Blood Substitutes
The 'blood substitutes' are really only "oxygen-carrying volume expanders"
that are being developed in order to replace red blood cell transfusion. They are however
finding other useful roles in patient care.
The products all have biological activity in terms of oxygen transport, the ability to
support life during severe anaemia and good volume resuscitation.
The challange is now to show clinical efficacy ie showing a clinical benefit to
patients.
- Decreasing the use of blood products and alleviating the red blood cell shortage.
- Decreasing transfusion related morbidity and mortality. When assessing this point it is
important to take into account not only the inherent risks of a blood transfusion, but
also the average person's risk of needing a blood transfusion.
- Easy use in logistically difficult environments
- Optimisation of oxygen transport - This will be the most complicated to prove because of
the incredibly efficient manner in which the human body deals with blood loss. The large
oxygen reserve present and the cardiovascular compensatory mechanisms to acute blood loss
in humans make the transfusion of red blood cells an incredibly contentious issue.
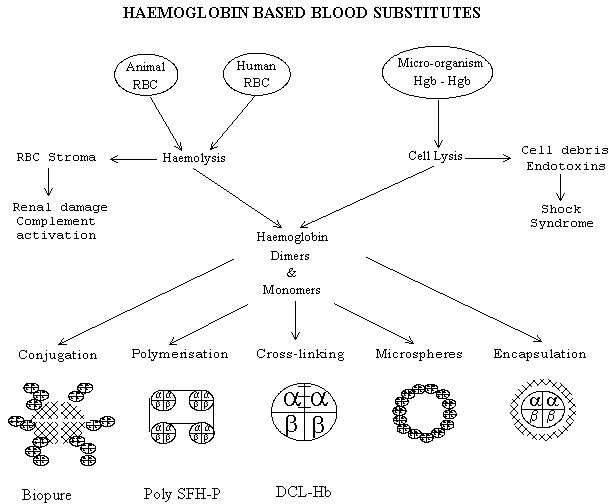
Classification of the 'blood substitutes'.
- Haemoglobin solutions
- Ultrastructural modification of the haemoglobin molecule
- Intramolecularly cross-linked haemoglobin - The tetramic structure is maintained by an
intramolecular cross-link between the two alpha or the two beta sub units.
- Polymerised haemoglobin - The formation of fumerate bridges between alpha molecules can
result in oligomes or polymers of haemoglobin.
- Conjugated haemoglobin - The linkage of free haemoglobin molecules to a soluble
non-haemoglobin polymer.
- Microsphere haemoglobin - Haemoglobin molecules are exposed to a high intensity
ultrasound which creates 'shells' of approximately one million haemoglobin molecules cross
linked by the superoxides formed during the sonication process.
- Liposome-encapsulation of haemoglobin - The typical form of synthetic liposome is a
phospholipid bilayer, with molecules of cholesterol added for increased rigidity and
mechanical stability. This liposome then encloses a stroma free haemoglobin solution and
2,3 DPG or inositol hexaphosphate as a gelatinous fluid.
- Perfluorocarbons - florinated organic solutions with a high solubility co-efficient for
oxygen
haemoglobin solutions
In assessing the currently availabe haemoglobin products, it is useful to compare them
to the ultimate 'ideal' "oxygen - carrying volume expander"
- A non toxic product with minimal possibility of disease transmission
Haemoglobin
based blood substitutes use human (expired donated blood), animal (bovine and transgenic
swine) and biotechnological (Escherichia coli and Sarcomyces cerevisiae) sources of
haemoglobin. There are potential logistic and safety problems associated with each of
these sources
- When haemoglobin is liberated from a cell (human red blood cells, swine red blood cells
or micro-organism) its tetramic structure (a 2 b 2 ) dissociates into
dimers and monomers that are filtered by the kidneys causing a diuresis and renal tubular
damage because of precipitation.
- All modifications of the haemoglobins result in a tetramer or a larger haemoglobin
molecule
- The residua of the red blood cell membrane, the stroma, contributes towards the renal
damage and causes activation of the complement cascade. The residua of the micro-organism
membrane acts like endotoxin and causes a toxic shock syndrome.
- All solutions are now exposed to an extensive pasturisation process
- The potential for interspecies disease transmission is poorly understood. Bovine
haemoglobin may transmit the prion responsible for bovine spongiform encephalitis.
- High oxygen-carrying capacity in a physiologically normal oxygen tension ie normal P 50
of 26.7mmHg
The haemoglobin solutions experience a marked left shift to a P 50 of
12-16mmHg, this limits oxygen unloading at the tissues and can contribute towards cellular
hypoxia.
- The oxygen binding co-operativity of haemoglobin is lost in the dimeric and monomeric
forms.
- Microbubble formation restores the oxygen affinity to close to normal. It also increases
the oxygen carrying capacity of the solution from 0.003mls/dl of plasma to 0.32ml/dl
- The loss of 2,3 - Diphosphoglycerate with the red blood cell stroma
- Piridoxal-5-phosphate can occupy the 2,3 - Diphosphoglycerate binding site and restore
the P 50 .
- Bovine haemoglobin utilises chloride ions to control its oxygen affinity
- The loss of adenosine triphosphate
- The relatively alkaline pH of plasma in comparison with the interior of the red blood
cells (the Bohr effect)
- The manufacture of genetically abnormal haemoglobin with a higher than normal P 50
(Hb Presbyterian) can counteract this.
- The encapsulation of haemoglobin solutions with 2,3 - Diphosphoglycerate or inositol
hexaphosphate can overcome this quite efficiently
- The oxidation to methaemoglobin
- No adverse effect on the normal cardiovascular compensation mechanism
- In normal physiology nitric oxide in the vessel wall is effective in maintaining
vasodilatation. Its effect is then terminated when it dissolves into the red blood cells
where it is scavenged by haemoglobin. The infusion of haemoglobin solutions is associated
with a pressor effect not influenced by the solution's volume and colloid osmotic
pressure. This additional pressor effect may be related to the binding of nitric oxide by
the free haemoglobin molecule within the plasma or within the vessel wall itself. The
haemoglobin molecule may also possess vasoconstricting properties.
- All these solutions improve the rheology of blood. They have no additional proteins, a
low viscosity, and improve blood flow within the smaller calibre vessels.
- No inherent properties that will limit the final concentration in humans
- When dealing with the concentrations of osmotically active particles, one osmole equals
the molecular weight of the substance in grams divided by the number of freely moving
particles each molecule liberates in solution. When infusing substances of 64 Kd a high
colloid osmotic pressure is exerted by these small molecules. This limits the final
concentration to ~7g/dl.
- The polymerisation of two to six tetramers can result in a molecule with a molecular
weight of 130-400kD and a lower colloid osmotic pressure
- Slow, efficient elimination from the intravascular compartment, resulting in a
clinically significant intravascular half life. Regeneration of native oxygen carrying
capacity by haematopoiesis often takes weeks or more.
- red blood cells transfused in the conventional manner have a 70% survival at 24 hours.
These red blood cells now have a "normal" life span of ~ 120 days.
- Free haemoglobin is rapidly combined with haptoglobin and cleared within the hour.
- The haemoglobin solutions are efficiently taken up by the reticulo-endothelial system.
This results in an intravascular retention time of 6-8 hours
- No interference with the coagulation system
- The haemoglobin solutions have minimal direct effect on coagulation. They do not
significantly alter the prothrombin time, partial thromboplastin time, factor X,
fibrinogen, antithrombin III, antiplasmin or plasminogen function.
- Haemoglobin solutions do not affect adenosine diphosphate-induced platelet aggregation
or platelet activation.
- No alteration of the immune system
- Purified haemoglobin solutions have little effect on complement activation. Endotoxins
liberated from micro-organism's cellular membrane do activate the complement cascade
- All the haemoglobin solutions are taken up by the reticulo-endothelial system. The more
complex solutions, liposome encapsulation, can disrupt the reticulo-endothelial system and
lead to reduced resistance to infections
- A long shelf half life
- Haemoglobin is oxidised to methaemoglobin when exposed to oxygen, it is then unable to
carry oxygen. The methaemoglobin reductase enzyme which is responsible for converting the
haemoglobin back, is not present in haemoglobin solutions.
- Storage in an oxygen free enviroment is not impossible
- Microbubbles undergo minimal degradation in solutions stored for 6 months at 4 o C.
- No physical or economic constraints on supply, allowing a large scale, affordable
production process.
- It has been estimated that 60 000 - 70 000 Kg would be needed to replace 10% of the
current red blood cell utilisation.
- As it is human blood that is in short supply, the outdated red blood cells are
decreasing in availability
- Bovine haemoglobin is relatively abundant - A 500Kg steer has 35L of blood of 12g/dl
haemoglobin which equals 4.2Kg per animal. Routinely phlebotomising a herd of 20 000
cattle should be immense fun!
- Transgenically manipulated pigs will have 50% of their haemoglobin identical to human
haemoglobin
- Universally compatible
- Unmodified, purified human haemoglobin that is free from the red blood cell components
has very limited immunogenicity. This allows a shift from the relatively abundant types of
cells (eg A) to universally compatible forms.
- The conjugation of haemoglobin to larger molecules further decreases the immunogenicity.
- Animal haemoglobins have been found to be weakly antigenic.
Products undergoing phase I and phase II clinical trials
- Bovine haemoglobin conjugated to polyethylene glycol, marketed as Biopure, has an
intravascular half life of 40-46 hours and a P 50 of 22mmHg
- Recombinant Hb1.1 - Hb Presbyterian made by transgenic E. Coli, yeast and mice, marketed
as Somatogen has a P 50 of 33mmHg
- Poly SFH - P haemoglobin manufactured by Northfield has piridoxal-5-phosphate occupying
the 2,3 Diphosphoglycerate binding sites and are cross linked into polymers with
glutaraldehyde. The intravascular half life is 40-46 hours and the the P 50 is
21-24mmHg
- Di-acytyl bis fumerate cross linked haemoglobin = DCL-Hb is manufactured by Baxter with
a fumerate link between the two alpha molecules. It has a plasma half life of 1.5-3 hours
and a P 50 of 29mmHg
Perfluorocarbons
Perfluorocarbons provide a fundamentally different and simpler approach to oxygen
transport. They are synthetic compounds composed of eight to ten carbon atoms that act as
solvents for oxygen molecules and obey Henry's law. The oxygen content relies on physical
solubilisation rather than on binding of the oxygen molecule.
The high oxygen and carbon dioxide dissolving capacities of fluorocarbons are related
to their elemental and physical nature. The electron-rich fluorine atoms tend to repel
each other, resulting in a very weak interaction between individual fluorocarbon molecules
in the liquid. These weak interactions yield a low surface tension and a high
compressibility. This facilitates the insertion of gas molecules.
The selection of an appropriate fluorocarbon is dependant on its vapour pressure. The
critical value is around 40 mmHg. Vapour pressures of 50 mmHg or more cause death in
mammals from massive gas emboli. Vapour pressures of below 30 mmHg result in prolonged
elimination from the body. Elimination is by vapourisation through the lungs and the skin
as these substances are not soluble in water and cannot be metabolised or excreted
unchanged in the kidneys.
Perfluorocarbons are completely immiscible in water and are administered as
microemulsions of small droplets 0.1-0.2mm in diameter dispersed in a physiological
solution.
- Egg yolk phospholipid, as found in total parenteral nutrition, is the safest emulsifier
- Poloxamers of the Pluronic type have also been used as emulsifiers but are less stable
(need to be stored frozen) and cannot be sterilised under standard conditions due to a low
cloud point (the temperature at which the emulsion will break down). They have also been
associated with complement activation
The smaller the size of the emulsion the higher the concentration of perfluorocarbon
solution possible. The small particle size also increases the overall perfluorocarbon /
plasma interface, increasing gas transport.
It is useful to compare the perfluorocarbons to an 'ideal' solution.
- A non toxic product with minimal possibility of disease transmission.
- Fluorocarbons are inert. There is no evidence of metabolism and they do not pose a
toxicological risk related to metabolic degradation. No complications have been found in
long term survivors after treatment with Fluosol ® or Oxygent ®.
Acute single dose toxicity studies have indicated a LD 50 of 55g/Kg body weight.
- When injected intravenously the emulsion droplets are taken up by the
reticulo-endothelial system which results in early effects, headache and lower backache,
and delayed (2-12hours) effects best described as 'flu like' including fever, chills and
nausea. All these effects are categorised as mild and are fully reversible within 12-24
hours, and can be attenuated by the prophylactic use of cyclo-oxygenase inhibitors
(ibuprofen) or corticosteroids (dexamethasone).
- High oxygen-carrying capacity in a physiologically normal oxygen tension ie normal P 50
of 26.7mmHg
- Fluorocarbons do effectively transport and deliver the expected amount of oxygen. This
oxygen is consumed by the tissues in preference to that carried by haemoglobin. The oxygen
is not bound to the carrier and so is readily available and virtually all of it is
extracted before the oxygen carried by haemoglobin begins to be released. Fluorocarbons
also appear to facilitate oxygen diffusion between red blood cells and the tissues,
especially during anaemia.
- The oxygen-carrying capacity per unit volume of a fluorocarbon emulsion depends on the
oxygen-dissolving capacity of the fluorocarbon utilised and on their concentration in the
emulsion. The concentration can be expressed in terms of weight of fluorocarbon to volume
of physiological solution (mg/dl) or in terms of volume of fluorocarbon to volume of
physiological solution. Fluosol ® DA is a 20% W/V or a 10% V/V solution.
- Plasma carriers 0.003ml of oxygen per 100ml of plasma for each mmHg of oxygen.
Fluorocarbons do not dissolve gases equally but the range is from 0.04-0.06ml of oxygen
per 100ml of compound for each mmHg of oxygen.
- At a P a O 2 of 100mmHg
- C a O 2 with an Hb of 14mg/dl = 20mls/dl
- Plasma = 0.3mls/dl
- 100% perfluorocarbon = 6mls/dl, Actual PFC-O 2 = (PFC O 2
solubility) x P a O 2 x fluorocrit
- No adverse effect on the normal cardiovascular compensation mechanism
- The microemulsion formulations decrease the viscosity, improve the rheology of blood and
improve microcirculatory flow
- No inherent properties that will limit the final concentration in humans
- Once the emulsion is injected into the vasculature it is the amount of fluorocarbon
administered that results in a certain concentration within the blood plasma compartment
(fluorocrit).
- The optimal concentration of fluorocarbon is 45-60% W/V as they are easy to infuse and
offer a great diversity in final fluorocrits.
- Slow, efficient elimination from the intravascular compartment, resulting in a
clinically significant intravascular half life. Regeneration of native oxygen carrying
capacity by haematopoiesis often takes weeks or more.
- The rapid uptake by the reticulo-endothelial system means that the oxygen-carrying
capacity provided will be lost from the circulation in ~1day.
- No interference with the coagulation system
- The perfluorocarbon solutions have minimal direct effect on coagulation. They do not
significantly alter the prothrombin time, partial thromboplastin time, factor X,
fibrinogen, antithrombin III, antiplasmin or plasminogen function.
- No alteration of the immune system
- Pluronic F-68 the emulsifier in Fluosol ® DA does activate the complement
cascade and has been associated with an anaphylactoid reaction.
- Massive uptake of these substances by the reticulo-endothelial system can lead to
reduced resistance to infections
- A long shelf half life
- The first generation of fluorocarbons required frozen storage and ultra sound mixing
prior to usage
- The second generation show marked stability from 4 o C to room temperature for
4 years.
- No physical or economic constraints on supply, allowing a large scale, affordable
production process.
- Fluorocarbons have been manufactured industrially in large tonnage since World Was II
when they were developed as part of the Manhattan project.
- Parenteral emulsion manufacturing technology has been used successfully for several
decades to produce large volumes of fat emulsions.
- Universally compatible
Currently available products
- Fluosol ® DA is a 20% weight:volume or a 10% volume:volume substance
- 14g Perfluorodecalin + 6g Perfluorotripropylamine = 20g compound in 100ml physiological
solution = Na, K, Cl, Ca, Mg, Bicarb, Glucose and hydroxyethyl starch
- It was used in an uncontrolled study in 1979 where the authors concluded that it was a
good volume expander but there was an absence of any discernible physiological benefit.
Analysis of the actual article however contradicts these conclusions.
- After the Fluosol infusion the oxygen extraction ratio improved and the fractional
inspired oxygen concentration could be lowered. This showed that Fluosol did deliver the
expected amount of oxygen and did unload it effectively. The patients died in the end not
because the fluorocarbons were ineffective but because an insufficient quantity (40ml/Kg)
of a dilute (20%) emulsion was administered, resulting in a low fluorocrit (3%) whose
limitation of intravascular half life (6-8hours) was not taken into account (only one
transfusion of Fluosol was given). Certainly the same volume of a 10% V/V suspension of
red blood cells would probably not have done better.
- Oxygent ™ is the best documented of the new emulsions. Perfluoro-octyl
bromide (perflubron) is lipophilic and rapidly excreted from the reticulo-endothelial
system and has a low enough vapour pressure and diffusibility in water to ensure emulsion
stability. It displays one of the highest oxygen dissolving capacities and can be made
into a 60% weight / volume ratio with egg yolk phospholipid as the emulsifier.
- Oxyflour ® (Supercytes ®) uses an undisclosed fluorocarbon in a
safflower oil in its formulation
Clinical applications
- Trauma
- Volume resuscitation following acute blood loss
- Peri-operative usage
- Haemodilution during pre-operative blood harvesting
- Intra-operative blood restoration
- The normal oxygen transport physiology permits rather extensive haemodilution. The
concept of using a diluent of low viscosity that also transports and releases oxygen, is
very attractive and makes the short intravascular half life an attractive feature
- Perfusion of ischaemic microvascular beds
- Acellular oxygen carriers can reach ischaemic tissues through the collateral
circulation.
- Peripheral vascular disease induced leg ulcers
- Treatment of the ischaemic crises in sickle-cell anaemia
- Perfusion of the tissues distal to balloon occlusion in coronary angioplasty limits
angina. The vasoconstrictive properties of the haemoglobin solutions have to be addressed
before they can be used in this vein.
- Cardioplegia solutions
- Extracorporeal circulation
- The low viscosity and robustness of these solutions is ideally suited for the harsh
conditions encounted in synthetic vessels
- Haemodialysis
- Cardiac bypass
- Perfusion of organs for transplantation
- Normothermic preservation with a perfusate that carries oxygen and carbon dioxide will
maintain aerobic metabolism and less damage to the organs will occur.
- Perfusion of devascularised tissues prior to revascularisation. This may also decrease
the systemic effects of reperfusion injuries.
- Extracorporeal oxygenation
- Increased transport of nitrogen. Nitrogen forms 70% of the air that is embolised. The
solubility of Nitrogen in perfluorocarbon is 10 000 times its solubility in plasma
- Prophylactic perfluorocarbons prevent the harmful effects of venous air embolism.
- Treatment of decompression sickness
- Increased oxygen delivery to radiosensitive tumours
- Radiotherapy and many anticancer drugs require that the tumour cells are well oxygenated
for maximal cytotoxicity.
- Transfusions in people with multiple antibodies
- Radiocontrast material
- A 90% W/V perfluorocarbon solution makes a very good angiographic contrast material
- The C-F bonds can be utilised by proper tuning of the radio frequency of a nuclear
magnetic resonance scanner.
- Treatment of the hypotension of septic shock
- It has been hypothesised that nitric oxide plays a major role in hypotension after
exposure to endotoxin. Once induced by cytokines, nitric oxide synthase continues to
produce nitric oxide even after the removal of the stimulus. The nitric oxide scavenging
effect of the haemoglobin solutions can be used to good effect in this model.
- Liquid ventilation
- The patient's lungs are filled with perfluorocarbon which eliminates the air-fluid
interface in the surfactant deficient alveoli. This eliminates the pulmonary surface
tension, improving lung compliance. The problem is that an apparatus must be used to
mechanically deliver and withdraw the liquid, purge the liquid of carbon dioxide, and
equilibrate with oxygen
- In a hybrid method, a perfluorocarbon is instilled into the lungs to replace functional
residual capacity and then the patient is placed on a standard mechanical ventilator that
delivers oxygen rich gas in tidal volumes.
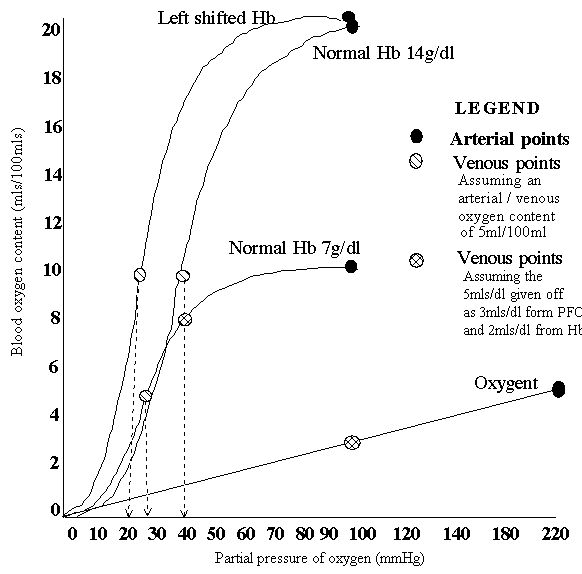
Influence of left shifting the oxygen haemoglobin curve and anaemia on the relationship
between oxygen tension and content. The venous oxygen tension is the major determinant of
anaerobic metabolism.
references
- Dietz N. Joyner M. Warner M. Blood substitutes: Fluids, Drugs, or Miracle Solutions?
Anaesthesia and Analgesia 1996; 82: 390-405.
- Zuck T. Riess J. Current status of injectable Oxygen Carriers. Critical Reviews in
Clinical Laboratory Sciences 1994; 31(4): 295-324.
- Spiess B. Perfluorocarbon Emulsions: One Approach to Intravenous Artificial Respiratory
Gas Transport. Annuals of anaesthesiology 1994; 11: 103-113.
- Nunn J. Nunn's applied respiratory physiology 4 th Edition 1993. Butterworth
Heinemann. Chapters 11:247-305.
- Sutcliffe A. Fluid resuscitation. current Opinion in Anaesthesiology 1996; 9:178-182
- Frey L. Messmer K. Transfusion therapy. Current Opinion in Anaesthesiolgy 1996;
9:183-187.
- Lech C. Fuhrman B. Morin F. Rath M. Perfluorocarbon associated gas exchange (partial
liquid ventilation) in respiratory distress syndrome: a prospective, randomised,
controlled study. Critical care medicine 1993, 21:1270-1278.
- Schreiber G. Busch M. Kleinman S. Korelitz J. The risk of transfusion-transmitted viral
infections. NEJM 334(26):1685-1691.
- Ulrich H. Kveten V. Pulmonary support - newer concepts and techniques. Current Opinion
in Anaesthesiology 1995, 8:132-138.
- Holland P. Viral infections and the blood supply. NEJM 1996 334(26): 1734-1735
- Gould, S. Rosen, A. Fluosol -DA as a red cell substitute in acute anaemia. NEJM; 1986;
314: 1653 - 1657.
- Tremper, K. Freidman, A. The preoperative treatment of severely anaemic patients with a
perfluorochemical oxygen transport fluid, Fluosol - DA. NEJM. 1982; 307: 277 - 283.
- Bridgewater, B. Recent advances in blood substitutes. Hospital update. 1994: 4 - 6.
- Tremper, M. Blood substitutes: what's needed, what's available and what will be
available. Refresher course lectures, San Francisco. 1994. 265.
- Winslow, M. Blood substitutes. Refresher course lectures, San Francisco. 1991. 222.
- Wall, S. Lipman, J. Kraus, P. Baragwanath ICU manual. Congress edition. 1992. Appendix.
- HAES- steril. Scientific product information. 1994.
- Haemaccel ® package insert.
- Stoelting, R. Pharmacology and physiology in anaesthetic practice. Second edition. J. B.
Lippincott company, Philadelphia. Chapter 36. 570 - 579.
- Ganong, W. Review of medical physiology. Sixteenth edition. Prentice - Hall
International , USA. Chapter 35. 604 - 610.



