This web page covers four main topics:
Note that it's traditional to draw a benzene ring with alternating double bonds - in CHIME you may be able to turn on/off the display of double bonds (Right click on the CHIME image for all the options). We have generally shown such rings with a contained circle - imagine the rings with a delocalised 'pi' distribution of electrons above and below the ring.
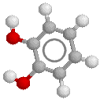
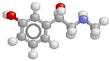
Here's the noradrenaline molecule
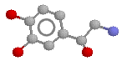
Noradrenaline is the adrenergic neurotransmitter. Therapeutically, as an inotrope and pressor agent it is a somewhat controversial drug, but some would regard it as the agent of choice in septic shock, given (of course) by continuous infusion. The potentially marked alpha agonist effect elicited by noradrenaline mandates cautious use, in an intensive care setting with appropriate monitoring. Note that septic patients often have profound vasodilatation and a blunted response to alpha agonists.
In our previous page, we discussed the synaptic vesicle cycle, and also the cycling of neurotransmitter release and uptake. We need to integrate this knowledge with the actual biosynthesis of catecholamines. Here are the synthetic pathways for noradrenaline, and adrenaline. (All images are CHIME clickable; carbon atoms are grey, oxygen red and nitrogen blue. Hydrogen atoms are not shown).
|
tyrosine | => |
dihydroxyphenylalanine (DOPA) | => |
dopamine | => |
noradrenaline | => |
adrenaline |
| Enzymes: |
|
|
|
|
The enzymes
Tyrosine hydroxylaseA pretty specific cytosolic enzyme that is the main control point for catecholamine synthesis - only found in catecholamine-containing cells. Noradrenaline inhibits the enzyme, providing feedback control. Potent inhibition also occurs with the analogue alpha-methyltyrosine. DOPA decarboxylaseThis widespread enzyme catalyses the next step in noradrenaline synthesis, with a host of other functions besides (decarboxylation of many other aromatic L-amino acids). dopamine ß-hydroxylaseFound in synaptic vesicles, this predominantly membrane-bound enzyme is actually released in tiny quantities when synaptic vesicles discharge (so a sensitive assay can give an idea of sympathetic activity)! As an aside, disulfiram inhibits the enzyme. (Examination candidates may wish to explore the challenge of giving an anaesthetic to someone on disulfiram)! phenylethanolamine N-methyl transferaseThis is an enzyme of the adrenal medulla whose production within 'A' cells is strangely enough modulated by adjacent adrenal steroid production! There is a small amount of the enzyme also present in certain areas of the brain! |
Note that the noradrenaline ==> adrenaline step occurs almost exclusively in the adrenal cortex, and not in (most) neurones. Once synthesized, noradrenaline is actively taken up into synaptic vesicles, using hydrogen ion gradients to drive uptake (The transport protein is similar to those that mediate reuptake from the synaptic cleft, but those transporters use sodium ion gradients). Noradrenaline content of most tissues is tightly controlled, so that as more is released, more is synthesized to compensate. In the mythical 'average man', it takes about ten hours for 'complete' turnover of noradrenaline stores! ATP is complexed with noradrenaline in the synaptic vesicle, and acts as a co-transmitter.
Noradrenaline release is profoundly influenced by homotropic and heterotropic regulation. Presynaptic alpha-2 receptors, when stimulated, inhibit its release, but other agents (acetylcholine, angiotensin II, prostaglandins, etc) also influence release.
Reserpine blocks transport of noradrenaline into presynaptic vesicles.
We have already discussed disposal of neurotransmitters released into the synaptic cleft - much noradrenaline is taken up by transporter proteins back into the presynaptic terminal. There is a long tradition of distinguishing between "uptake 1 " and "uptake 2 ", terms respectively applied to our by now well-known neuronal uptake, and uptake into cells that are not neurones (uptake 2 is "extraneuronal").
The pumpsUptake 1 is very important in the re-cycling of noradrenaline, while uptake 2 simply mops up the rest. Vesicular uptakeThere appear to be two distinct vesicular amine transporters, called VAT1 and VAT2. Presynaptic uptake - 'uptake 1'This noradrenaline transporter protein ('NET') was discussed in our previous lecture. 'Uptake 2'Quantitatively, uptake 2 gobbles up a lot more neurotransmitter into smooth and cardiac muscle and even the endothelium. As one might expect, uptake 1 is particularly good at grabbing noradrenaline; uptake 2 is more specific for adrenaline. |
Now that we've briefly covered synthesis (and release), let's consider removal of noradrenaline from the synaptic cleft. In the peripheral nervous system, neuronal reuptake is the major mechanism for terminating noradrenaline's action at the synapse. Things are different in the central nervous system , where a degradative enzyme is pretty important in dealing with noradrenaline. The enzyme is monoamine oxidase (MAO). Hence the previous importance of MAO inhibitors in management of depressive disorders in man.
There is yet another enzyme, catechol O-methyl transferase (COMT) that is widespread both inside and outside neuronal tissue. It inactivates both noradrenaline and adrenaline.
There are several metabolic steps in the activation of catecholamines, and the amount of end-product depends on where the catecholamines are being broken down. CNS noradrenaline is predominantly metabolised to 3 methoxy 4 hydroxy phenylglycol (MOPEG), while peripherally the same transmitter is metabolised to a heady cocktail that includes a fair amount of vanillylmandelic acid (VMA). Traditionally we have measured urinary VMA to diagnose phaeochromocytoma, but we now know that this is a pretty insensitive test (and thus rely on a combination of clinical suspicion, urinary metanephrines, and possibly blood levels of catecholamines too). For completeness, here are the main noradrenaline degradative pathways. The enzymes are COMT, MAO, aldehyde dehydrogenase (ADH) and aldehyde reductase (AR).
noradrenaline [NA]
/ \
(COMT)/ \(MAO)
/ \
normetanephrine [NM] NA aldehyde
\ / \ \
(MAO) \ (COMT)/ \(AR) \(ADH)
\ / \ \
NM aldehyde DOPEG \
\ \ / \
\ (AR)\ /(COMT) \
\ \ / \
\ MOPEG DOMA
\ /
(ADH)\ /(COMT)
\ /
\ /
vanillylmandelic acid
The importance of Monoamine oxidase - MAOExamination candidates are still often asked about MAO inhibitors (MAOI) - this may be because such questions illustrate important principles within the autonomic nervous system. These agents were very popular years ago for treatment of depression, but potentially life-threatening drug interactions made them rather unpopular. One may occasionally encounter MAOI - phenelzine, tranylcypromine, and isocarboxazid. In addition to their unfortunate interaction with tyramine in foods, MAOI have other undesirable effects, including the occurrence of fatal interaction when used with opioids (notably pethidine), tricyclic antidepressants (TCAs), and selective serotonin uptake inhibitors; and profound CNS depression when used with alcohol, or general anaesthetics. Exam candidates might wish to explore the "neuroleptic malignant syndrome" that occurs when MAOIs interact with, for example, TCAs. This would appear to be a heterogeneous syndrome, and the interaction with pethidine, for example, seems quite distinct from that with TCAs. Selegiline is an interesting MAOI in that it specifically inhibits only one of the two MAO isoforms - MAO-B, which degrades dopamine. The drug is thus useful in Parkinson's disease. |
We already know about the synthesis of adrenaline in the suprarenal gland. Removal of adrenaline is similar to noradrenaline degradation, but uptake-1 is not important. Both COMT and MAO act on adrenaline. Metabolites are analogous to those produced during noradrenaline metabolism.
Adrenaline is well-recognised as an important agent in resuscitation notably following cardiac arrest, and with severe anaphylaxis. Recently, the use of high-dose adrenaline has been frowned upon, and other agents such as vasopressin may eventually partially supplant it. (Amiodarone also deserves mention in this setting). Adrenaline has been disparaged as an inotrope in septic shock for a variety of reasons, including its propensity to raise lactate levels, but this latter effect does not necessarily carry the same grave prognosis seen with other causes of hyperlactataemia. Dosage should rapidly be titrated to effect, as discussed in our page on inotropic support.
Uptake-1 inhibitors like cocaine will greatly enhance the magnitude of the response to administered adrenaline, as well as the duration of this response. Alpha blockade will 'unmask' predominant beta effects, and conversely, ß blockade will permit nasty unopposed alpha effects (think phaeochromocytoma)! Arrhythmias (related to both endogenous and exogenous adrenaline) are a well-recognised complication of halothane administration, related to halothane's sensitizing effect on the myocardium, which is made even worse by hypocapnia.
All adrenergic receptors work via G proteins. The end results of stimulation of the different receptors are however profoundly different. Beta receptors stimulate adenylate cyclase resulting in increased intracellular levels of cyclic AMP; alpha-1 receptors cause release of intracellular calcium (via Phospholipase C), and stimulation of alpha-2 receptors lowers the activity of adenylate cyclase.
We will discuss:
(Note that some authorities prefer to regard alpha-1, alpha-2 and beta receptors as being on the same footing, and then talk about the subtypes)It's important to note that much of the work on adrenergic receptors was done on laboratory animals such as rats. These animals are quite different from man - for example, in the rat, cardiac beta receptors are beta-1. In man, there also numerous beta-2 and beta-3 adrenoceptors in the myocardium, although we're not clear how important they are here!
The alpha-1 and alpha-2 receptors both have well-characterised subtypes, confirmed by gene sequencing. These include:
Alpha-1A receptors mediate positive inotropic responses in the heart, and are also mainly responsible for cardiac hypertrophy ! [Can J Physiol Pharmacol 2000 Apr;78(4):267-92]. They also mediate vasoconstriction and metabolic effects of alpha-1 receptor stimulation. They are coupled via Gp/q to phospholipase C, D and A2 (we will abbreviate these to PLC, PLD, PLA2). Alpha-1B receptors appear mainly to activate phospholipase C, resulting in production of IP3 and DAG from PIP2, with a consequent rise in intracellular calcium ion concentration. Alpha-1D receptors are probably only important in vasoconstriction. Stimulation of PLC is greatest for alpha-1A (> alpha-1B > alpha-1D). [Eur J Pharmacol 1999 Jun 30;375(1-3):261-76]. Splice variants of alpha-1A have been described.
When stimulated, alpha-2 receptors lower cAMP levels by inhibiting
adenyl cyclase, an action mediated by G i . This is probably
not the whole story, with other G proteins probably mediating effects.
The major alpha-2 receptor in the spinal cord appears to be alpha-2A.
Alpha 2A receptors mediate central anti-hypertensive effects; alpha
2C receptors are also important in homotropic regulation, and
alpha 2B receptor stimulation may cause vasoconstriction (mediating
the trasient peripheral vasoconstriction seen when IV alpha-2 agonists
are given).
Genetic polymorphism in these receptors is well-documented.
Alpha-1 receptors
These receptors are commonly found on smooth muscle, and generally
cause contraction of the muscle (but may relax smooth muscle
in the gastrointestinal tract). They have a few other effects -
liver glycogen is broken down when they are stimulated, and production
of saliva is increased. (Note that adrenaline-induced hyperglycaemia
is contributed to by alpha receptors, as beta blockade alone doesn't
obliterate this hyperglycaemic response).
adrenaline = noradrenaline >>>isoprenaline
(also the selective agonists phenylephrine and oxymetazoline)
ergotamine
prazosin, doxazocin are selective antagonists
Alpha-2 receptors
Alpha-2 receptors are pretty different from alpha-1. They are important
in homotropic regulation of neurotransmitter release at adrenergic
synaptic junctions, but in addition are found on platelets (mediating
aggregation), and beta cells in the pancreas (inhibiting insulin secretion).
These receptors do have something in common with alpha-1 receptors, in
that both are found on vascular smooth muscle, and participate in
vasoconstriction.
adrenaline = noradrenaline >>> isoprenaline
(also the selective agonist clenbuterol; and alpha-methylnoradrenaline; and other agents such as dexmedetomidine, and mivazerol; etomidate and pethidine have some alpha-2 agonist activity. )
clonidine is a selective agonist.
yohimbine, idazoxan are alpha-2 selective antagonists, as are atipamezole, efaroxan and rauwolscine. BRL44408 and BRL48962 antagonise alpha-2A receptors, imiloxan alpha-2B.
The structure and function of the beta-1 and beta-2 receptors have been well characterised. Seven-spanning transmembrane proteins, they transduce signals using G proteins, notably G s . In many ways they are strangely similar to muscarinic receptors! All beta receptors increase cellular levels of cyclic AMP. ß-adrenergic receptor kinase modifies their function.
Beta receptors change both in numbers and responsiveness to stimulation quite quickly. Denervation or blockade rapidly increase numbers; conversely, prolonged stimulation results in down regulation of the response to beta agonists, especially at ß-1 receptors. The latter 'desensitisation' is complex, perhaps related to:
Beta-1 receptors also increase amylase secretion from the salivary glands.
isoprenaline > adrenaline = noradrenaline
(also dobutamine, xamoterol: specific for ß-1)
dichloroisoprenaline (the first 'beta blocker'); the 'blockers' oxprenolol and alprenolol actually have significant partial agonist activity.
A whole slew of beta blockers {see below}, but beta-1 selective
are atenolol and metoprolol.
Beta-2 receptors - not for noradrenaline
ß-2 receptors can be neatly contrasted with alpha-1 receptors,
in that the former generally relax smooth muscle, wherever they
are found.
In addition, they have several metabolic effects,
causing breakdown of glycogen
in
both liver and skeletal muscle.
Remember that noradrenaline has minimal ß-2 effect.
One useful effect of beta-2 receptor stimulation is inhibition of release of histamine (and other granule components) from mast cells. Lymphocytes are also inhibited by ß-2 agonists, although the clinical importance of this effect is far from clear.
Beta-2 receptor stimulation also increases the tension generated in fast-twitch skeletal muscle fibres (especially if they're tired). Changes in muscle kinetics combined with a (probable) effect on the muscle spindle probably contribute to the tremor seen with administration of beta-2 agonists like adrenaline. Other longer-term effects probably account for the improvement in rate and force of muscle contraction seen with beta agonists like clenbuterol (used as an illegal 'performance-enhancing' agent by some 'sportsmen').
It is thought that the reduction in portal venous pressure seen with propranolol or nadolol therapy is mainly related to blockade of beta-2 receptors. This is useful in management of portal hypetension. An even more pronounced effect has been reported with carvedilol 12.5mg daily [Aliment Pharmacol Ther 2002 Mar;16(3):373-80].
isoprenaline > adrenaline >> noradrenaline
selective are salbutamol, terbutaline, salmeterol
butoxamine
Beta-3 receptors
These receptors have been best characterised in brown fat, where
they mediate thermogenesis (in infants and hibernating animals).
We now know that they are also present in sites such
as the myocardium, where they may antagonise the effects of
beta-1 and beta-2 receptor stimulation, although such speculation
may be simplistic [Circulation 2001 Nov 13;104(20):2485-91]! It is also thought that
(unlike ß-1 receptors) they do not undergo down-regulation
with long-term stimulation.
The role of beta-3 receptors in the white adipose tissue of
rats is well established, but controversial in humans - the advent
of specific human ß-3 blockers may clear things up
[J Pharmacol Exp Ther 1999 Aug;290(2):649-55].
isoprenaline = noradrenaline > adrenaline
(BRL 37344 is specific, at least in rats)
Have been described (L-748,328 and L-748,337); most conventional beta-blockers have no effect on beta-3 receptors.
After we've considered how drug structure influences actions, we'll look at:
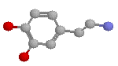
After much tinkering, drug designers have become fairly smart at predicting the effects of various drugs on receptors. They've coined the name "structure activity relationships" for the 'rules' they've derived. Here are a few of the generalisations they've come up with, when it comes to modifying the noradrenaline molecule, especially as regards effects at beta receptors (They're fairly hopeless for predicting alpha effects, on the main). Here's the noradrenaline molecule again, followed by a list of modifications and their effects: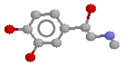
Above we discussed adrenaline and noradrenaline, and we have previously covered the distribution of adrenergic receptors in the various tissues. We know the various effects of stimulation of the several types and sub-types of adrenergic receptor, and structure-activity relationships for the various agents. We will thus confine our further discussion of adrenergic agonists to specific drugs:
This agent acts at alpha, beta and dopaminergic receptors; it even has an indirect noradrenaline-releasing effect! It has traditionally been taught that it improves mesenteric blood flow in shock; this simplistic view is not supported by any substantial evidence! There are also still intensive care units around the world where dopamine is administered in a ritualistic fashion to 'improve renal function', a practice based more on myth than science. Some authorities regard dopamine as the initial drug of choice in states such as septic shock.
The half-life of dopamine in the circulation is short (about 1 minute). Again, it is traditionally taught that low doses (~3 µg/kg/min) have predominant dopaminergic effects, intermediate doses beta effects, and higher doses (> 10µg/kg/min) predominant alpha effects, but such differentiation may not be encountered clinically.
Dopamine receptors are not without interest. Five receptors have been cloned; the most important are D1 and D2. (Otherwise known as DA1 and DA2). The D5 receptor is like D1, the others like D2. DA1 receptors are post-synaptic, and prominently present in the smooth muscle of the kidneys and mesenteric vessels. DA2 receptors are presynaptic, and appear to modulate noradrenaline release. Stimulation of central DA2 receptors causes emesis (cf. eg. apomorphine).
We have briefly reviewed this agent elsewhere, (concentrating on its misuse in "splanchnic resuscitation"). In summary, dobutamine is a racemic mixture with good beta-1 agonist activity, and minuscule agonist effects on beta-2 receptors. Unfortunately, it doesn't stop there, for (-)dobutamine is a partial agonist at alpha receptors, and (+)dobutamine has insignificant alpha agonist effects. Overall, in the presence of other alpha agonists, we might expect dobutamine to act as an alpha blocker! In addition, dobutamine antagonises the beta effects of adrenaline both in vitro and in vivo.
The principle use of dobutamine appears to be in short-term support
of patients with heart-failure, where it can indeed be extremely useful.
Here's the molecule:
Consult our brief review of dopexamine here! Dopexamine has both dopaminergic and ß-2 agonist effects, and fenoldopam is a selective agonist at DA1 receptors.
A relatively pure, non-selective beta agonist, this is now an 'orphan' drug. Isoprenaline is a vital drug in the management of paediatric cardiac patients. Adult clinical use is limited to increasing heart rate as a temporising measure in patients with complete heart block (while preparing for pacing), although even this use has fallen into disfavour. Metabolism is by COMT, with no uptake-1.
"Beta-2 selective" (but only partially so, and remember that the heart
also has beta-2 receptors), this agent finds its main use as an aerosol
in asthma. Terbutaline and albuterol are similar. ß-2 agonists
have also been used as tocolytics. Side effects include tachycardia,
arrhythmias, and hypokalaemia.

Salmeterol represents an interesting wrinkle in beta-2 therapy of
asthma. A long-acting beta agonist, salmeterol has a long lipophilic
side chain that binds to an 'exosite' near the beta receptor with
high affinity. This binding results in sustained (over 12 hour) receptor
stimulation, with substantial clinical improvement in symptom control.
Salmeterol is transformed by CYP 3A4 to the alpha-hydroxy derivative
which is eliminated in the faeces.
This is a selective alpha-1 agonist. It has rapid
onset, and short duration of action (5-10 min) when given IV, so
infusion is the best method of administration. The agent is logically
used to provide vasoconstriction where there is hypotension due to
vasodilatation, for example due to neuraxial block. (Phenylephrine
is also used as a mydriatic and nasal decongestant).
Methoxamine has similar alpha-1 activity, but longer duration of
action (30-60 min).
Clonidine is the prototypic alpha-2 agonist. It's main anti-hypertensive
action appears to be due to central alpha-2 receptor stimulation (with
lowered sympathetic outflow). It has also been used in treatment of
a wide variety of disorders, such as chronic pain, withdrawal syndromes,
management of nausea, treatment of diarrhoea in diabetic with
autonomic neuropathy, and postmenopausal symptoms.
In addition, alpha-2 agonists may be used as anaesthetic
adjuvants. One such agent is dexmedetomidine..
There is a vast array of agents now available for blocking adrenergic
receptors. Most block either alpha or beta receptors, although some
are widely touted as agents that block both (for example, labetalol,
which is a beta blocker with relatively modest alpha-blocking effects).
Phenylephrine
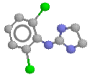
Clonidine
Dexmedetomidine
The alpha-2 agonists are potentially very useful agents in anaesthesia.
Drugs such as xylidine have been used for years in veterinary anaesthesia,
and now we have dexmedetomidine, which we have
reviewed previously - also look
here. Alpha-2 agonists seem to be a favoured
topic in recent post-graduate anaesthetic exams.
Adrenergic receptor blockers
Alpha blockers
These may be non-selective (the older agents
phenoxybenzamine and phentolamine), or selective. Note that the nonselective
agents are largely obsolete because of the high rate of side effects, notably
postural hypotension and tachycardia; phenoxybenzamine irreversibly
binds alpha receptors
and is thus still, in the opinion of some, a good agent for pre-operative
management of phaeochromocytoma.
(Roisen's criteria are traditionally used to establish the presence
of effective blockade). Once a decent degree
of alpha blockade has been established, then only may beta blockade
be instituted in phaeochromocytoma (Consider why this should be)!
The various alpha-1 and alpha-2 selective alpha blockers are considered below.
Note that alpha blockers seem to be falling into disfavour for the management of hypertension, especially in patients with associated heart failure (largely because the doxazosin arm of the ALLHAT study was stopped due to an increased incidence of heart failure and cardiovascular events).
Prazosin is a selective, reversible alpha-1 blocker, and has been
used to manage hypertension, and even in pre-operative preparation
of patients with phaeochromocytoma. It should not be used in heart
failure.
Other similar agents include doxazosin, terazosin.
This agent is 'super-selective', having a preference for alpha 1A
and alpha 1D receptors. Such selectivity may be of value as these receptors are
prominent in the bladder neck, rendering tamsulosin useful in management
of lower urinary tract symptoms. See [Drugs 2002;62(1):135-67].
Agents such as yohimbine and idazoxan are mainly experimental curiosities
used to analyse alpha receptor subtypes.
Prazosin
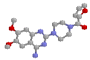
Tamsulosin
Alpha-2 blockers
Beta blockers
Many beta blockers such as propranolol block beta-1 and beta-2 receptors equally.
Exceptions are the ß-1 selective agents atenolol and metoprolol.
(The first beta-1 selective agent was practolol, but it unfortunately
caused fibrosing syndromes, and has been abandoned).
The ways that beta-blockers combat hypertension are still poorly understood. Possible mechanisms are:
Beta blockers are also of great use in management of ischaemic heart disease, mainly through lowering of myocardial oxygen demand. Control of heart rate is an important effect, as tachycardia selectively lessens the relative duration of diastole, and as we know, coronary flow is mainly in diastole. Several studies now attest to the protective effect of beta-blockers in the peri-operative period, in patients with underlying ischaemic heart disease. The mechanism of this observed effect is still obscure. Conversely, it is generally extremely unwise to stop beta-blockers suddenly, especially in the peri-operative period, as there is a risk of tachycardia and even myocardial infarction.
Beta blockade is also useful in management of thyrotoxicosis, hypertension, migraine headaches (prophylaxis), hypertrophic cardiomyopathy, and certain arrhythmias.
Of fairly recent interest is the observation that heart failure is a hyper-adrenergic state. Careful introduction of beta-blockade in patients in heart failure seems paradoxical but probably has survival benefits! Carvedilol has featured prominently in this regard.
Side effects of beta-blockers can largely be predicted through knowledge of where beta receptors are present. These agents may precipitate life-threatening bronchospasm in asthmatics, and they should be used with extreme caution in patients in heart failure (but see above)! We are now much less jumpy about appropriate use in diabetics (despite a theoretical propensity to mask the symptoms of hypoglycaemia) and there seems to be scant evidence that beta blockers worsen limb ischaemia in patients with peripheral vascular disease. The bradycardic effects of beta blockers may be a problem, and are even occasionally life threatening, making them contra-indicated in patients with heart block. One reason why beta blockers are unpopular in young active persons with hypertension is the tiredness that may occur. Lipophilic beta blockers may be associated with particularly vivid nightmares.
There is confusion in the literature as to whether this magical property of some beta blockers (such as pindolol and acebutolol) is merely a manifestation of partial agonist properties, or whether it is mediated by other mechanisms. The bottom line is that such agents appear to cause less bradycardia than agents devoid of "ISA". Other ß-blockers with ISA are carteolol, celiprolol, dilevalol, oxprenolol and penbutolol.
These are insignificant for most beta blockers, although "quinidine-like" effects have been claimed for propranolol and acebutolol.
Many beta blockers are rather lipid soluble. These (such as propranolol, metoprolol and pindolol) are rapidly metabolised in the liver, mainly by CYP 2D6, although the attendant problems seen with other drugs and this enzyme appear less common with beta blockers, although dose requirements may vary considerably between individuals. Half-lives are generally short unless controlled-release formulations are used.
In contrast, the water-soluble beta blockers (atenolol, sotalol and nadolol) have longer half-lives and are mainly renally excreted. CNS penetration is lower, with fewer CNS side effects such as depression and nightmares.
There's a confusing array of these agents, including propranolol, nadolol, pindolol, sotalol, oxprenolol and timolol. From a practical (and examination) point of view, it's probably important to know the following well:

These include atenolol and metoprolol (as well as betaxolol, bevantolol, and perhaps esmolol).
This beta blocker lacks ISA and MSA; one can deduce its metabolism and short half-life from its lipophilicity. Some slow-release formulations of metoprolol have peculiar doses.
Examination candidates should probably know this agent fairly well. Important topics to discuss would be its water solubility, metabolism, ß-1 selectivity, and recent studies showing improved post-operative survival even after very short-term use in the peri-operative period. Here's some information on Mangano's papers.
This beta blocker is relatively beta-1 selective. hydrolysis by nonspecific esterases accounts for its tiny half-life of about 9 minutes. The traditional approach of starting therapy with a 0.5mg/kg IV bolus is excessive, silly, and potentially life-threatening. Be more gentle. This agent has been much abused to "manage tachycardia" intra-operatively, where the anaesthetist should rather be thinking about the cause of the tachycardia, for example, pain)!
There are several agents that by devious means interfere with noradrenergic transmission. Here are a few mechanisms by which they work:
This experimental agent is taken up by noradrenergic terminals - its toxic metabolites then then destroys these, causing "chemical sympathectomy".
Binds the vesicle noradrenaline transporter and blocks noradrenaline
uptake. An anti-hypertensive, obsolete because of its main side-effect
of depression.
As mentioned above, reserpine depletes neuronal stores of noradrenaline in synaptic vesicles. There are other groups of drugs that interfere with the vesicles in interesting ways:
Drugs such as guanethidine , another obsolete anti-hypertensive, potently inhibit release of noradrenaline from sympathetic nerve terminals. The mechanisms are complex including (a) initial uptake by uptake-1; (b) concentration in vesicles, with displacement of noradrenaline from the vesicles, and possibly even (c) structural damage to sympathetic neurones.
(Bretylium is taken up in a similar fashion to guanethidine,
but initially releases noradrenaline, before causing profound block
of noradrenaline release, without depletion of noradrenaline stores.
It has vanishingly small utility as an anti-arrhythmic, formerly
often administered as the "last rites" in terminal ventricular
arrhythmias)!
These agents are sitting ducks for drug interaction:
Of particular 'classical' importance is the interaction between MAOI and the indirectly acting agent tyramine , found in a host of foods such as parmesan cheese and Chianti. Tyramine is normally removed by MAO in the gut. You now understand how this combination may produce fatal hypertension.
Amphetamine acts in a similar fashion .. one only has to contemplate
the mechanism of action of such drugs to see why tolerance to their
effects develops with time. Here's the amphetamine molecule again:
See our introductory page.
The classical agents here are the tricyclic antidepressants - they inhibit the noradrenaline re-uptake pump. An example is desipramine :
Cocaine has similar actions with increased sympathetic outflow, and massive potentiation of the effect of noradrenaline:
Other agents also interfere with uptake-1. They include amphetamine, phenoxybenzamine and guanethidine.
 Chime - you must
first register to download the software, but it's free.
Chime - you must
first register to download the software, but it's free.
 ACP Journal
club review of one of Mangano's papers. (N Engl J Med. 1996 Dec 5;335:1713-20).
Here's a
ACP Journal
club review of one of Mangano's papers. (N Engl J Med. 1996 Dec 5;335:1713-20).
Here's a  follow up paper (Anesthesiology 1998 Jan;88(1):7-17)
follow up paper (Anesthesiology 1998 Jan;88(1):7-17)| Date of First Publication: 2002/7/8 | Date of Last Update: 2006/10/24 | Web page author: Click here |