The physical distance between Europe and North America is perhaps a few thousand kilometers but from a acid base point of view, we are talking light years. It is sad that acid-base (which should be relatively easily understood, now that we have modern computers to do the dirty work for us) is plagued by unnecessary argument. Particularly vituperative comments have come from Severinghaus and Siggaard-Andersen, criticising the work of Stewart and the "strong ion difference" concept. We believe that although Siggaard-Andersen has made a massive contribution to the understanding of acid-base, Stewart has provided a sound mathematical approach which is of great use in understanding acid-base. This article briefly examines Peter Stewart's approach to acid-base. It's best read carefully, but you can jump straight to the calculator, a Java applet that implements his model!
This web page makes few assumptions about your knowledge. It should be easily understood by anyone with a high-school education and a bit of a background in clinical medicine and physiology. We try to keep things simple - if you feel that our tone is patronising, then you are probably too advanced to be reading such simple stuff! Note that a bit of spadework is still required to work through the maths - pen and paper remain a wonderful medium for this! Because solutions for the more complex equations require the use of computers, we use a tiny little bit of "computerspeak" - as little as we can get away with! We do however feel obliged to use a star (*) to indicate multiplication and a slash (/) for division. This page does not assume that you can speak a computer language.
Remember from physiology that if you have two substances A and B reversibly combining to form substance C, that when the reaction is at equilibrium the equation governing the equilibrium is:
where K is the rate constant for the reaction.
Throughout the body pH is vitally important. Anyone who has a blood pH of below 7.0 is likely to die soon if this isn't corrected. Likewise, a pH of over 7.6 is frequently associated with serious illness and death. Remember that pH is the log of the reciprocal of the hydrogen ion concentration. Why use such a convoluted measure, and not just talk about the concentration of hydrogen ions? The answer is mainly historical but also perhaps reflects the ongoing inability of humans to cope with concepts that cannot be counted on fingers (with perhaps a little help from the toes)! Rather talk about pH 7 than 1 * 10-7 mol/litre, or (god forbid!) 100 nanomol/l of hydrogen ion.
Unfortunately, such terminology has only served to complicate things,
especially when we start plotting how pH changes when we change another
ion concentration - we have to continually remember that we are dealing
with non-linear axes on any graph we might draw. We will try and clear
up some of the confusion. It goes without saying that in any solution
mass is conserved (the amount
of each component remains constant unless some of that substance is
added or removed, either physically or by participating in a chemical
reaction), and that any aqueous solution is
electrically neutral (the
number of positive ions equals the number of negative ones). But let's
review a few other fundamental definitions:
We define an acidic solution as one that has a higher concentration of
hydrogen ions than of hydroxyl ions. A basic solution is the reverse of
this, and a solution is acid-base neutral in the rare circumstance where
the concentrations are exactly the same! Simplistically, we then say
that an acid is something that makes a solution more acidic,
while a base does the opposite! Note that already the Scandinavian
hackles rise, as they fashionably follow Bronsted with his definition of
an acid as a "proton donor" and so on - but we have done nothing wicked -
we simply use a convenient definition that will have great utility in
the clinical arena. We are not pooh-poohing Bronsted, we are simply applying
a useful label!
Another convenience is to categorise substances that dissociate when put
into water (electrolytes) into two groups. If they dissociate almost totally
in the pH range we are interested in, we call them strong electrolytes -
if only partially dissociated, they are weak. Note that we will
also refer to ion concentrations in units of moles of charge rather
than moles of substance, as we are after all more interested in the
ionic (charge) properties of the substance than actual concentrations.
For example, if each ion has a charge of +2 (say Calcium) then there
are twice as many moles of charge as there are moles of calcium.
A convenient shorthand is use of the term normal,
for example if we have 1 mole of Sodium Sulphate in a litre of water,
then the solution is 2 normal in the sodium ion (there are
two mol of Na+ per litre), as well as being 2 normal in sulphate
(there is only one mole of SO4-- but it has a charge
of 2-).
Also note that technically we should be talking about "effective
concentration" (or "activity") of an ion rather than concentration,
as the two may differ. But for most ions in physiological solutions,
the difference is not significant.
Here is a list of common strong ions (in biological solutions):
In all biology, approximations are necessary. Although each of the
perhaps 1014 cells in the body is unique, we can define groups
of cells and treat them as single entities. Likewise, we have identifiable
subsets of body fluids that we can define and analyse. Important components
are
Water is weird. This weirdness is the basis of life. Among its most peculiar
characteristics are:
The last statement deserves a bit of explanation. Because water
molecules are small (molecular weight of just 16+1+1 = 18) the molar
concentration of water is enormous - about 55.3 mol/litre at 37 degrees C.
There are a billion times fewer hydrogen ions than there are water
molecules in a glass of water. Remember that even the hydrogen ion is
a convenient fiction - it's just a simple symbol (H+) for
a statistically very complex association between a proton and a whole
lot of surrounding water molecules that the proton finds very attractive.
The life of the common proton is far more complex than even the most
Byzantine modern TV soap opera!
Water dissociates as follows:
This is a very rapid reaction indeed, and we will always assume that
equilibrium is reached instantaneously in biological solutions.
At equilibrium, the following equation must hold:
As we've already implied by saying that water doesn't dissociate very
much, the dissociation constant KW is rather small, in fact, about
4.3 * 10-16Eq/l at 37o Celsius. This is really tiny.
Also note that KW varies rather remarkably with temperature - for example
at 25 C it is about 1.8 * 10-16Eq/l ! If you're smart, you'll
immediately realise that the pH of a glass of water changes dramatically
as its temperature changes! Further contemplation will also assure you that
we can assume a new constant
As an aside, you can approximate the value of KW' by:
KW' = 8.754 * 10-10 * e(-1.01*106) / T2)
From the above we know that
What is an acidic solution?
What is a strong electrolyte?
Weak electrolytes (at physiological pH) include the carbon dioxide /
bicarbonate system, and reactions involving the proteins found in
some body fluids.
What body compartments are there?
We will concentrate on the first five 'compartments'.
..but of course there is also..
1. A simple case - Pure Water
H2O <<<====> H+ + OH-
[H+] * [OH-] = KW * [H2O]
because the tiny changes in water concentration will be totally insignificant
compared with the total amount of water present.
KW' = KW * [H2O]
KW' is
just a convenient label for the product of the two constants, KW
and the molar concentration of water.
where temperature T is expressed in degrees Kelvin Okay, so how do we find the pH of pure water?
But it is clear that even if we know KW', we still need to
find one of the other variables, the hydroxyl ion concentration, if we
are to determine the hydrogen ion concentration. Fortunately, in pure
water the only ions present are hydrogen ion and hydroxyl ion, so if
the water is to be electrically neutral then:
[H+] * [OH-] = KW'
A solution is basic if [H+] < ROOT (KW')
Take some water. Add strong electrolytes, such as NaOH and HCl, which we know will almost completely dissociate. We now have a heady mix of water, Na+, Cl-, H+ and OH- ions. What will happen to the hydrogen ion concentration in this mix?
We already know that water dissociation constrains us to:
If SID is positive and bigger than about 10-6 Eq/l you can see that KW becomes insignificant, and Equation #3 becomes very nearly the same as:
If the SID is negative and bigger than about 10-6 Eq/l then Equation #2 simplifies out to: [H+] = -[SID] but such solutions are not commonly encountered in biological systems.
"Okay, this is all very well" you say "but what is the practical significance of all this maths?" Easy. The above tells us that in a solution containing strong ions, if you want to calculate the pH, you must:
You can also see that if we have (for example) an acidic solution and we progressively add base, there will be a sudden, rapid rise in pH as we approach the point where [SID] is zero, and then add just a tiny bit more base. Consider the following diagram (our applet) showing how pH changes with SID - Press the button!
pH is easily measured so now we finally understand the concept of "titratable acidity" of for example urine (we add base until there is a sudden rise in pH and this is the point where "titration is complete" - that is, the SID of the solution is zero). But remember that this doesn't tell us what titratable acidity means, and also note that the "sudden change in pH" is an artefact induced because pH is logarithmic: nothing that dramatic happens if we look at actual hydrogen ion concentration! Try this - in the above applet, change the Y axis parameter from pH to [H+] and press on the Calculate button again!
It is instructive to read Stewart's book where he looks at titration of interstitial pH using HCl - the counter-intuitive nature of pH is clearly seen.
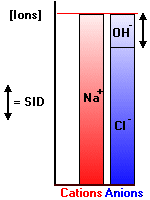
Electrical neutrality in solution demands that Equation #1 is satisfied - the sum of [negative] and [positive] ions is always zero. This is conveniently represented by two adjacent bar plots as shown in the following illustration (after Stewart, p43).

The solution is a simple one containing [H+], [OH-], [Na+] and [Cl-]. The bar plot (Gamblegram) clearly shows the disposition of ions. The minuscule amount of H+ ion is not shown. Such plots can be used for far more complex solutions, often with unmeasured ions (where the term "gap" is often used, as in "anion gap"). It is crystal clear from the plot that the SID is equal to the amount of hydroxyl ion. In alkaline solutions the SID will be positive, in acidic, negative.
Adding a weak electrolyte (one that only partially dissociates in the
pH range we are considering) complicates things rather a lot. In body
fluids such as plasma, the most important weak electrolyte is albumin,
but the principles hold for all weak electrolytes.
Let's look at the case of a weak acid, HA (such as albumin) that dissociates to form
H+ and A-, as follows:
As above, we can work
things out if we have the correct numbers, and plug them into the relevant
equations. Two of the equations we already know, those governing the
dissociation of water and the requirement for electrical neutrality
(slightly modified to include the dissociated anion A-,
derived from the acid):
[H+] + [OH-] + [SID] + [A-]
= 0 .. Equation #1A
[HA] + [A-] = [ATOT]
.. Equation #5
Unfortunately for people such as myself who are used to counting
on their fingers, the solution
of the above four equations is based on cubic equations. Fortunately,
when we use digital computers to solve such equations, the difficulty
evaporates! We explore this below.
When we actually go to the bother of solving the equations, we reach
several initially rather counter-intuitive conclusions.
Click on Calculate and then change the Albumin value to say
40, and click once more. The difference between the two curves is clearly
seen - albumin only really has an effect between an SID of zero and an
SID of about 0.02. (An albumen of 40 g/litre more-or-less corresponds
to an ATOT of 0.016 Eq/l, perhaps a bit less)!
Click on the above graph to get the picture with an albumin of 40g/l,
and then decrease the albumin to 0 and click again - notice how the
new, red, albumin-free graph changes LESS steeply! Wham!
Calculations for a weak base are similar to the case of the weak acid
above. In an analogous way, added weak base is most effective in the
SID range zero to -[BTOT]. The difference is that buffering
here does occur: the base flattens the [H+] versus [SID]
curve very effectively!
If you've successfully come this far, the following section should
not be beyond your capabilities, even if computers scare you witless!
You may however skip the following computerese.
If you choose to read it, then also get a piece of paper and write
down the equations, and work things out.
We take the equations from above:
[H+] + [OH-] + [SID]
+ [A-] = 0
.. Equation #1A
[H+] * [A-] = KA
* [HA]
.. Equation #4
[HA] + [A-] = [ATOT]
.. Equation #5
[HA] = [ATOT] - [A-]
(from Equation #5)
and by substituting the latter into Equation #4,
Many people are terrified of computers - an unnecessary state of mind,
as all computers do is give us a way of implementing an algorithm like
the one above in a fast and accurate way!
In order to implement our algorithm we need to express it in
an unambiguous way. We use statements written in a computer
language to do so.
We will now 'formalise' the above algorithm. We won't yet write
it in a specific computer language, we will just make things more
precise, without (we hope) compromising readability. Our only
assumptions are:
If you were unwise enough to read the above in one sitting, we
suggest that you take a tea break to marshal your resources!
Remember the fundamentals:
People who are familiar with acid base as it is commonly taught, usually
cut their teeth on the Henderson-Hasselbalch equation. There is little
wrong with the H-H equation, other than the fact that it only represents
part of the truth. Here we start to explore how carbon dioxide
behaves, but in what we regard as the proper context. Much of the
following may seem familiar, but be careful - don't lose sight of
the big picture!
Take our familiar mixture of strong ions and water, and
expose it to CO2. What happens?
Four things can happen to CO2 gas when exposed to water -
it can dissolve, react with water to form carbonic acid, or even form
bicarbonate or carbonate ions. We will explore each of these in turn, but
the two most significant reactions are the formation of carbonate and
bicarbonate, as each has its own equilibrium constant. By now you will
realise that these reactions with their equilibrium constants will have
a profound influence on the whole system, and it is only
in the context of the whole system that we can understand the
role of carbon dioxide. Let's see:
[CO2(dissolved)]
= SCO2 * PCO2
.. Equation #7A
For Kf/Kr we have substituted
SCO2, otherwise called the Solubility of CO2.
In other words, the amount of dissolved CO2 depends
on the partial pressure of CO2 times a rate constant.
SCO2 is dependent on temperature, and at 37 C it
is about 3.0 * 10-5 Eq/litre/mmHg.
Note that if we
are examining a fluid that isn't in contact with gas, we still
talk about a partial pressure of that gas in solution "as if"
it were exposed to, and in equilibrium with, a gas containing
that gas at that partial pressure. This is just another convenient
way of representing the concentration of dissolved gas in solution.
The value of KH at 37 C is 9 * 10-8 Eq/litre -
because of this, the H2CO3 concentration is
far smaller than the amount of dissolved CO2.
The reaction of CO2 with water is
SLOW , with a half time of about 30 seconds, fortunately
speeded up to microseconds by the carbonic anhydrase abundantly present
in most tissues.
It follows that:
[H+] * [HCO3-] =
KC * PCO2
.. Equation #8
A good value for KC is 2.6 * 10-11
(Eq/l)2/mmHg.
A typical value for K3 is 6 * 10-11 Eq/litre.
As usual, we need four simultaneous equations to work out all the dependent
variables (given the independent ones). These are our old familiar equation
#0, Equations #8 and #9, and the requirement for electrical neutrality:
[H+] * [HCO3-] =
KC * PCO2
.. Equation #8
[H+] * [CO32-] =
K3 * [HCO3-]
.. Equation #9
[SID] + [H+] - [OH-] - [HCO3-]
- [CO32-] = 0
.. Equation #10
It is important to distinguish between dependent and independent
variables. Bicarbonate concentration and hydrogen ion concentration
are dependent variables, but SID and pCO2 are independent!
We work out our equation for [H+] in the same way we
did in Section 3 above, and solve it using a computer, again as above.
On contemplating the results thus obtained, a whole host of interesting
conclusions emerge. These include:
The combination of strong ions, carbon dioxide and a weak acid
closely models blood plasma, but also provides a fairly
accurate representation of intracellular fluids. Blood plasma is rich
in weak acids, the majority being proteins. For the purposes of analysis
it is probably moderately accurate to regard them as being all one acid
with a single ATOT and single KA. This assumption
is not however central to Stewart's work - as is seen in the articles
by Figge et al who extend his model with multiple
Ka's.
Following the pattern we established in previous sections, we
identify the independent variables ( [SID],
PCO2, ATOT which
are respectively the strong ion difference, partial pressure of CO2
and the total amount of weak acid present). In addition, we need to know
KW', KA, KC and K3. Given these,
we can calculate any one of eight dependent variables: Exactly as we have done before, we derive six simultaneous equations,
most of which are old friends:
[H+] * [A-] = KA * [HA]
.. Equation #4
[HA] + [A-] = [ATOT]
.. Equation #5
[H+] * [HCO3-] =
KC * PCO2
.. Equation #8
[H+] * [CO32-] =
K3 * [HCO3-]
.. Equation #9
and finally, to maintain electrical neutrality:A weak acid
HA <===> H+ + A-
The other two equations are not too difficult, as they are based on the dissociation of
the acid, and the necessity for conservation of the total amount of acid,
which we abbreviate to ATOT. These
are (in turn):
[H+] * [OH-] = KW'
.. Equation #0
[H+] * [A-] = KA * [HA]
.. Equation #4
It is best to play extensively with the actual equations in order to
see how truly non-intuitive their effects are! Remember that in order
to predict any result of altering the nature of a strong ion solution
containing a weak acid, we need to know:
On the other hand, with [SID] > [ATOT],
OH- have to fill the gap between the two (for [A-]
can't of course exceed [ATOT]), so [OH-] =
[SID] - [ATOT]. Consider the following:
Without even one of these four, you are stymied, and cannot for example
predict pH. Note that in this example, (as in all the others on this page)
we have dependent and independent variables. Things that
we change by external maneuvers (such as [SID] or [ATOT])
are independent variables. Values that respond to such changes
(e.g. pH) are dependent variables.
WE MUST KNOW ALL THE INDEPENDENT VARIABLES
IN ORDER TO CALCULATE THE DEPENDENT ONES!
A weak base
Solving equations using computers
Our approach:
[H+] * [OH-] = KW'
.. Equation #0
we can derive that:
[OH-] = KW' / [H+]
(from Equation #0)
[A-] = Ka * [ATOT] / ( [H+] + KA )
A program to do this
TEA BREAK!
4. Strong ions with Carbon dioxide
Forward Reaction
Reverse Reaction
===> Depends on partial pressure of CO2
<=== Depends on concentration of dissolved CO2
Rate of forward reaction = Kf * PCO2
Rate of reverse reaction = Kr * [CO2
(dissolved)]
..AT EQUILIBRIUM..
Equilibrium is represented by:
If we treat [h30] as constant, and rearrange things a bit we get:
Equilibrium is represented by:
Equilibrium is represented by:
The big picture - finding the pH!
[H+] * [OH-] = KW'
.. Equation #0
and a Weak Acid
HCO3-, A-, HA, CO2
(dissolved),
CO32-, H2CO3, OH-,
and H+. Note that dissolved CO2 and H2CO3
are easily determined from Equations #7A and #7B.
The equation that we derive
in an analogous fashion to our method above where we discussed
a computer solution is..
[H+] * [OH-] = KW'
.. Equation #0
[SID] + [H+] - [HCO3-] -
[A-] - [CO32-] - [OH-]
= 0
Try experimenting further with our Java applet for blood plasma. This applet uses the above equation and iterative approach. First, simply press the "calculate" button. Then play - try seeing what happens to pH as you vary the SID and Albumin level. Then change the independent variable (on the X axis). Later you can see how other dependent variables (eg CO3--) change with variations in e.g. pCO2.
ionz - Copyright (C) J van Schalkwyk, 1999
I'd like to thank everyone for their feedback (mainly positive) about
this page, particularly Rinaldo Bellomo and
John Kellum, who have
contributed enormously to spreading the news about the physicochemical
approach, and also Jon Waters, and
 Philip Watson.
A good website that explores both the traditional, and now the Stewart approach,
is that of Kerry Brandis.
Philip Watson.
A good website that explores both the traditional, and now the Stewart approach,
is that of Kerry Brandis.
Thanks too to PJ Hilton, who pointed out a typo in Stewart's book, which we carried over in a mildly embarrassing fashion.
Recent developments are explored on yet another page on anaesthetist.com. Further constructive comment is of course still welcome. By the way the actual distance between Brown University (Stewart) and Copenhagen (Siggaard-Andersen) is 5958 km, just for the record.
It's a tragedy that Peter Stewart is dead - the man was a genius}
| Date of First Publication: 1999 | Date of Last Update: 2006/10/24 | Web page author: Click here |