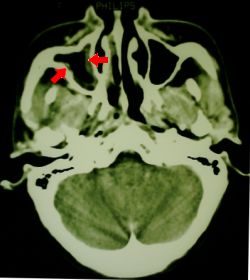
Fig 1. CT scan showing sinusitis
Mucormycosis is a rare but serious fungal infection that rapidly attacks and usually kills its untreated victims, who are often acidotic, 'immunocompromised', or receiving desferrioxamine. Many reported cases have been poorly controlled diabetics. 'Synonyms' are zygomycosis or phycomycosis. The presumed way in which the fungus attacks compromised individuals is fascinating. Iron availability limits the growth of many microorganisms, and this seems to be the case for the phycomycete fungi that cause mucormycosis. Normally, the fungal hyphae produce a substance called rhizoferrin, which binds iron avidly. The iron-rhizoferrin complex is then taken back into the fungus, and the iron becomes available for vital intracellular processes. Human resistance to fungal infection seems to depend to a large degree on non-immune factors. One of these defence strategies is to limit iron availability to invading microorganisms, by binding the iron to proteins such as apotransferrin. It is clear that a defect in the body's ability to hide iron from invading fungi will predispose to overwhelming fungal attack.
A convincing experiment by Artis and colleagues in 1982 supported this hypothesis. They took serum from seven patients with diabetic ketoacidosis, and showed that four of the seven sera supported profuse growth of Rhizopus oryzae, a common cause of mucormycosis, at a pH under 7.3, which pH one might expect in the blood and tissues of a ketoacidotic diabetic. This breach in the body's defences seemed to be related to a decreased ability of the sera to bind iron at low pH! (Diabetes 1982 Dec;31(12):1109-14 ) Such defects were not seen in normal sera. Phycomycetes can grow amazingly fast. This has been shown in experiments which looked at Rhizopus as a potential source of protein - it has been estimated that 450kg of fungus, provided with adequate substrate, can produce up to 40kg of (dry weight) protein per hour ! (Appl Environ Microbiol 1976 Sep;32(3):381-7 ) It is thus not surprising that given the right conditions, mucormycosis can within a day destroy the sinuses and invade the brain of a susceptible person.A poorly-controlled diabetic in her twenties was admitted with ketoacidosis to the medical ward of a third-world teaching hospital. On admission, she had a vague localised right lower lobe infiltrate on chest x-ray, and mild headache. It was presumed that pneumonia had precipitated her ketoacidosis, and a combination of amoxycillin + clavulanic acid and gentamicin was started. Fluid resuscitation and insulin were commenced.
During her stay in the ward, her severe acidosis was not controlled (possibly related to inadequate fluid administration). There was a slight worsening of the radiological findings on chest x-ray. No pathogenic organism had been cultured from blood or sputum. She was admitted to intensive care where her ketoacidosis promptly resolved in response to rehydration and continuous infusion of insulin. A quinolone antibiotic was started.
Two days later, just prior to discharge from intensive care, onset of swelling of the right side of the face was noted, accompanied by local sinus tenderness. An urgent 'ENT' consult was obtained, as the head of ICU was concerned about possible mucormycosis. The ENT surgeon said that on examination he could find no evidence of mucor, (although he did note some "dried blood" on the turbinates) and dismissed the diagnosis. Nevertheless, an urgent CT scan was obtained, which merely showed a 'mild to moderate sinusitis' with no evidence of bone, orbital, or brain invasion. The patient was discharged to the medical ward looking well, still with mild erythema over the right side of the face, and mild chemosis. Metronidazole was added to her antibiotic regimen, and she was started on amphotericin B, 40mg/day, because of ongoing concern about a fungal infection.

Fig 1. CT scan showing sinusitis
Thirty six hours later, she was again in florid ketoacidosis. Careful examination showed an area of black discolouration on her hard palate. Microscopy of a nasal scraping showed phycomycete fungal elements. She was taken to theatre and an extensive debridement and resection was performed, with preservation of the right eye. Tissue specimens were sent for mycology and histology.
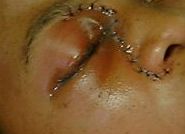
Fig 2. Eye of patient with mucor (post-operatively), showing periorbital oedema
Her ketoacidosis was aggressively controlled, and her pH brought back to normal. The amphotericin dosage was increased to 60mg/day. Fungal cultures grew a Rhizopus species. After a relook operation and prolonged stay in ICU, she was discharged with a residual sixth nerve palsy involving the right eye. Despite a further increase in her amphotericin dosage to 75mg/day, her renal function was unaffected. Her right lower lobe infiltrate resolved - no fungal infection was ever confirmed at this site. She was discharged from hospital after a month of high-dose amphotericin therapy, with no apparent residual fungal infection.
Microscopy of these fungi is characteristic - the hyphae are broad, branch at ninety degrees, and are non-septate. There seems to be some controversy over the classification of the different types - most mycologists seem to regard R oryzae and R arrhizus as the same species, for example.
From our introduction, it is clear that persons with long-standing acidosis are predisposed, especially if they are iron-overloaded. As expected, apart from poorly controlled diabetics , the following individuals are likely to acquire this rare disease:
The chelation therapy is interesting. The reason why patients treated on desferrioxamine B (DFO) get fungal infection is that the DFO makes iron available to the fungus even at very low iron concentrations! (de Lockt &c : Biochem Pharmacol 1994 May 18;47(10):1843-50; Verdonck : Mycoses 1993 Jan-Feb;36(1-2):9-12 ) Shortened survival has been shown in guinea pigs with experimental mucormycosis when given deferoxamine (Kidney Int 1989 Dec;36(6):1061-8 ), and in mice given iron and/or deferoxamine (Mycopathologia 1990 May;110(2):87-91 ).
Many classify skin infections separately, as 'entomophthoromycosis'. The phycomycete Basidiobolus microsporus is often implicated in this unpronounceable condition.
Suspect the disease. A delay of even twelve hours in diagnosis may be fatal. Important points in diagnosis are:
If there is a clinical suspicion of mucormycosis, sufficient tissue should be obtained immediately from the site of infection and examined for the characteristic fungal elements. Extensive local resection should then be performed without delay.
The mainstay of therapy is aggressive surgical resection of the whole lesion. This may involve disfiguring surgery. Some have gone so far as to recommend frozen sections during surgery to ensure that the resection margins are clear (Kimura &c Arch Pathol Lab Med 1999 May;123(5):386-90 ). Surgery should always be instituted without delay in acute rhinocerebral mucormycosis . The role of surgery in pulmonary mucormycosis is not clear, but some would advocate resection.
On diagnosis, intravenous amphotericin B should be started at at least 1mg/kg. This should be administered for a long period of time - most would recommend several months, or more. The usual precautions for amphotericin administration should be observed. Nephrotoxicity is a major worry. Anecdotal evidence suggests that newer, less toxic forms of amphotericin such as liposomal amphotericin (AmBisome), Amphotec (colloidal dispersion), or possibly even amphotericin B lipid complex (Abelcet) may be beneficial if the patient is developing substantial side effects related to amphotericin administration, or there is central nervous system involvement. Such agents are expensive, but may be effective even with intracerebral extension (Strasser &c : Arch Intern Med 1996 Feb 12;156(3):337-9 ).
One should probably resist the temptation to add in other antifungals, as their value is unproven. Some Rhizopus species are resistant to azoles such as fluconazole (Chemotherapy 1989;35(4):267-72 ), and even more worrying is the extensive evidence that there is antagonism between these agents in vitro (although to our knowledge this has not been shown in vivo).
Interstitial injection of amphotericin, intraventricular administration, and use of Ommaya reservoirs in abscess cavities and/or in the ventricles have been used (Neurosurgery 1998 Mar;42(3):644-8 ).
The therapeutic role of hyperbaric oxygen, and G-CSF (see eg Fukushima : Cancer 1995 Sep 1;76(5):895-9 ) is not clear. One report suggested that local application of half-strength hydrogen peroxide may be of benefit (Va Med Q 1996 Winter;123(1):30-2 ), after resection.
The prognosis of the disease surely depends on how early and aggressively it is managed. Owing to the rarity of mucormycosis, few substantial studies exist and there are no studies where treatment was for example randomised to different modalities (It is difficult to conceive an ethically acceptable study that could do this). Response to treatment could be better characterised if there was some type of staging system that indicated severity (for example, extent of tissue involvement, proximity to the brain, or presence or absence of cerebral involvement).
Most of the above references were found on PubMed.
Rewarding search strategies on general-purpose search engines
such as Altavista use search terms such as:
Rhizopus AND (mucormycosis OR zygomycosis)
or perhaps
Rhizopus AND Absidia AND Apophysomyces
Most of the web pages are simply case reports or brief mentions of the disease, but the following are of some interest:
 Good pictures on
an ophthalmology site;
Good pictures on
an ophthalmology site;
 Absidia, with pictures;
Absidia, with pictures;
 hyperbaric oxygen therapy. (You'll need to be a MedScape subscriber);
hyperbaric oxygen therapy. (You'll need to be a MedScape subscriber);
 here (with a pretty picture of the non-pathogenic Pilobolus).
here (with a pretty picture of the non-pathogenic Pilobolus).
| Date of First Publication: 27 December 1999 | Date of Last Update: 2006/10/24 | Web page author: Click here |