Dose: Usually 6mg/kg/day, after two loading doses 12hr apart (each 6mg/kg)
|
|
|
|
Dose: Usually 6mg/kg/day, after two loading doses 12hr apart (each 6mg/kg) | ||
Teicoplanin is mainly used in moderate to severe infections with Gram-positive organisms. It is not effective against Gram-negatives. It is important to remember that in Gram-positive infections that are known to be sensitive to simples agent such as penicillin or cloxacillin, these are the drugs of choice. As is always the case, the correct drug should be given for the correct duration, to minimise resistance. The following microorganisms are usually or frequently sensitive to teicoplanin:
There is a small number of organisms that are inherently resistant to teicoplanin. These are usually not pathogenic, or not often encountered, but include:
Far more important is acquired resistance . This has recently emerged as a substantial problem in Enterocci . Organisms that are resistant to teicoplanin are also often resistant to Vancomycin, and vice versa. Some organisms may be sensitive to teicoplanin, and resistant to vancomycin, or the reverse may occur. If vancomycin and teicoplanin resistance is present, then there may be no effective treatment for the patient, apart from some experimental agents that are not yet readily available.
Dosing details are considered below. The conventional dose is 6mg/kg/day (400mg/day in a 70kg adult) following one or more loading doses, all given intravenously. The only reason for oral administration would be Clostridium difficile-induced enterocolitis, as the drug is very poorly absorbed orally.
Teicoplanin is extremely useful in the following clinical situations:
The drug may also be useful in:
Patients with the following infections may require higher than conventional doses (ie over 6mg/kg/day, following loading dose(s)):
It is clearly not appropriate to use intravenous or intramuscular teicoplanin if:

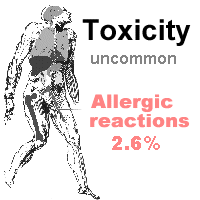
Side effects are relatively uncommon, and the side-effect profile is favourable, especially when compared with vancomycin. Side effects include:

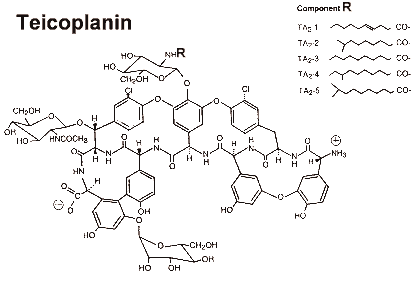
Teicoplanin is actually a group of at least six bulky glycopeptides, all with very similar activities. The main components are termed A2-1 to A2-5, and A3-1.
The drug may be given intravenously (IV) or intramuscularly (IM), but
is poorly absorbed orally. Peak blood concentrations after IV administration
are achieved after half an hour. Given IM, levels peak at about four hours.
For best antimicrobial effect, authorities recommend that the levels
remain above 10mg/Litre. This is usually achieved with the following
dosage regimen (in a 70kg adult):
Absorption
It is NOT necessary to monitor serum levels unless there is severe renal dysfunction.
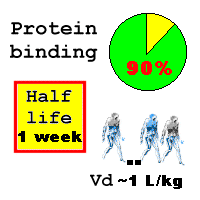
The volume of distribution is normally between 0.8 L/kg and 1.6 L/kg. Protein binding is high (90% in serum, mainly to albumin), and good levels (up to three times serum peak levels) are achieved in heart muscle. Penetration into fat and cerebrospinal fluid is poor. Bone penetration of teicoplanin is good (better than vancomycin) [ Drago L, et al. Drugs Exp Clin Res 1998;24(4):185-90 ] The vitreous humour is poorly penetrated [ Eye 1998;12 ( Pt 2):252-5 ]
The body doesn't metabolise teicoplanin substantially (only 3%) - it is mainly excreted in the urine. Metabolites have little antibacterial activity. The terminal half life is long , about one week (155-168 hours) and increases as renal function declines. There are minor differences in the handling of the five main components of teicoplanin, of no clinical significance.
With renal dysfunction:
Higher doses (12mg/kg/day) are indicated with:
Teicoplanin inhibits formation of cell walls in Gram-positive bacteria. It does this by interfering with formation of links in the cell wall (transglycosylation), acting on amino acyl-D-alanyl-D-alanine residues. In "sensitive" organisms, it inhibits bacterial growth at levels under 8mg/Litre, killing these organisms at higher concentrations. If the organisms are exposed to the agent for longer periods of time, then killing occurs more effectively! In some (but possibly not all) bacterial populations, a post-antibiotic effect has been seen, this lasting for up to over four hours.
Despite the increasing prevalence of glycopeptide resistance, a recent large study still showed that most Gram-positive clinical isolates are sensitive [ Int J Antimicrob Agents 1998 Nov;10(4):271-7 ]. Resistance to teicoplanin often implies resistance to vancomycin and vice versa. Glycopeptide resistance has been divided into:
 Tn1546,
and shows up as high-level resistance to both teicoplanin
and vancomycin.
Tn1546,
and shows up as high-level resistance to both teicoplanin
and vancomycin.It is thought by some that administration of the drug avoparcin
to livestock and birds as an antibiotic growth promoter is one reason for the rise
in antimicrobial resistance in Enterococci. A recent article
from the
New England Journal of Medicine
supports this, finding glycopeptide
resistance commonly present in turkeys given avoparcin. Avoparcin acts
at the same site as teicoplanin and vancomycin, and has been temporarily
banned in Europe because of these suspicions. See a recent article
in the  New Scientist.
New Scientist.
Of extreme concern is the recent emergence of decreased sensitivity of Staphylococcus aureus to vancomycin. This resistance is not related to the VanA..VanC genes, but we continue to worry that S aureus might just acquire for example the VanA gene. Amazingly enough, scientists have been rash (mad?) enough to experimentally transfer VanA into S. aureus!
There are several investigational drugs that may eventually become clinically useful with teicoplanin resistance, however these are not yet available. They include streptogramins (quinupristin/dalfopristin), some fluoroquinolones (clinafloxacin, grepafloxacin, moxifloxacin, gatifloxacin, and trovafloxacin) as well as everninomicin derivatives (SCH27899), ketolides, and oxazolidinones (linezolid), [ Jones RN et al, Diagn Microbiol Infect Dis 1999 Feb;33(2):101-12 ], and possibly the glycopeptide LY333328.
Relatively few studies have compared clinical efficacy of vancomycin with that of teicoplanin. The following general conclusions appear to hold:
Synergy may occur with:
Information on this page is provided for teaching purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.
| Date of First Publication: 7 June 1999 | Date of Last Update: 2006/10/24 | Web page author: Click here |