Clinical Applications of Coagulation
(Perioperative and ICU Concerns)
|
|
| List of Abbreviations |
| ATIII |
Antithrombin III |
| GIT |
Gastrointestinal Tract |
| aPTT |
activated Partial Thromboplastin Time |
| ACT |
Activated Clotting Time |
| PIVKA |
Proteins Induced by Vitamin K Absence |
| INR |
International Normalised Ratio |
| LMWH |
Low Molecular Weight Heparin |
| UFH |
Unfractionated Heparin |
| DVT |
Venous Thromboembolism |
|
Management of patients presenting for surgery on full warfarin
anticoagulation is controversial. The warfarin will be for three basic reasons, deep
vein thrombosis prevention, prevention of atrial thrombosis in atrial fibrillation and the
prevention of thrombosis on a mechanical valve.
A coagulation profile that is as near to normal as possible is needed to prevent
excess bleeding from any type of surgery. This must be balanced against removing the
anticoagulation which increases the risk of thrombosis. The inherent risk of venous
thrombosis present with all surgery will be increased in patients who have had a previous
deep vein thrombosis. The risk of arterial thrombosis will revert back to that seen
in a non anticoagulated setting.
The indication for the anticoagulation, the pre-operative risk for thrombosis (venous
and arterial) and the risks of postoperative bleeding must be weighed carefully in all
patients. Essentially we should decide between two different management strategies.
High Risk of Pre-operative Thrombosis
- Venous thrombosis
- An acute episode of Deep vein thrombosis within the last three months
- Multiple previous acute episodes of deep vein thrombosis
- Hereditary thrombophilic state
- Active carcinoma
- Atrial/arterial thrombosis
- Clinical criteria
Previous cerebrovascular accident
Female over the age of 75 years
Hypertensive patients
Left ventricular dysfunction
Congestive Cardiac Failure
- Trans-oesophageal criteria
Complex aortic plaque
Existing left atrial thrombus
Dense spontaneous echocardiographic contrast
- Mechanical Heart Valve
- Previous valvular thrombosis
- Caged-ball (Star-Edwards) and tilt (Bjork-Shiley) type valves in the mitral position
A protocol driven, perioperative Heparin infusion will prevent a significant number of fatal and
serious complications that would arise in a non-anticoagulated patient.
Low risk of peri-operative thrombosis
- Warfarin is stopped 3 days prior to surgery
- INR must be <1.5 prior to surgery
- Additional prophylaxis against deep vein thrombosis is instituted peri-operatively
- Pneumatic compression of the lower legs
- Low molecular weight heparin is given
- Warfarin is restarted as soon as possible after surgery
Patients who are on low dose subcutaneous heparin or low molecular weight heparin
for deep vein thrombosis prophylaxis and patients who are on low dose aspirin therapy are
not considered to be at an increased risk of epidural or subarachnoid haematoma formation.
- Wille-Jorgensen, P. Jorgensen, L. Lumbar regional anaesthesia and
prophylactic anticoagulant therapy; is the combination safe? Anaesthesia 1989; 46:
623-627.
- Vandermeulen, E. van Aken, H. Anticoagulants and spinal-epidural
anaesthesia. Anesth Analg 1994; 79: 1165-1177.
- Berqvist, D. Lindblad, B. Risk of combining low molecular weight heparin for
thromboprophylaxis and epidural or spinal anaesthesia. Semin Thromb Hemost 1993; 19
Suppl. 1: 147-151.
- Wolf, H. Experience with regional anaesthesia in patients receiving low molecular
weight heparins. Semin Thromb Hemost 1993; 19 Suppl. 1: 152-154
- de Sweit, M. Redman, C. Aspirin, extradural anaesthesia and the MRC
collaborative low-dose aspirin study in pregnancy (CLASP). [letter] Br j Anasthe
1992: 69: 109.
Patients on full heparin anticoagulation or who will be placed on full heparin
anticoagulation intra-operatively may have a rachidial block provided the following
criteria are adhered to.
- There must be a clear benefit of regional anaesthesia for the patient
- The surgery can be delayed if there is a "bloody tap" during the block.
- The aPTT must be documented as normal prior to insertion of the block
- The heparin may only be started a minimum of 2 hours after the block
- An epidural catheter can only be removed during a break in anticoagulation
- 4 hours after stopping a heparin infusion
- 2 hours prior to restarting a heparin infusion
- The patient is monitored frequently in the post operative period for neurological
impairment and back pain.
- Vandermeulen, E. van Aken, H. Anticoagulants and spinal-epidural
anaesthesia. Anesth Analg 1994; 79: 1165-1177.
Action
Enhancement of the action of the serine protease inhibitors AT III and heparin co
factor II.
A specific 5 sequence sugar is responsible for the binding of heparin to AT III
and an exponential increase in its antifactor Xa activity, a further 13 sugars are needed
to cross link the AT III and thrombin in order to increase ATIII's antithrombin action
Heparin does not inhibit thrombin bound to fibrin or Xa bound to platelets.
Unfractioned heparin has a Xa:Thrombin inhibiting effect of 1:1.
The low molecular weight heparins have varying Xa:Thrombin inhibiting effects
Dalteparin - Fragmin 2.7:1
Enoxaparin - Clexane 3.8:1
Heparin causes the release of extrinsic pathway inhibitor
Unfractionated and low molecular weight heparins release extrinsic pathway
inhibitor from the endothelium and enhance its inhibitory activity against factor Xa and
VIIIa.
Biokinetics
Absorption
- 30% of a subcutaneous dose of unfractionated heparin is absorbed
- 80% or more of a subcutaneous dose of low molecular weight heparin is absorbed,
reflecting less binding to the endothelium.
Distribution
- Unfractionated heparin is highly protein bound to all the acute phase reactant proteins
with a volume of distribution equal to whole body water (600ml/Kg) due to endothelial cell
binding
- Heparin binds avidly to the plastic of infusion sets
- Low molecular weight heparin binds less to plasma proteins and the acute phase
reactants, resulting in a more predictable anticoagulant response.
Metabolism
- Unfractionated heparin has a rapid redistribution phase followed by a saturatable zero
order elimination phase t1/2 life ~2hours
- Low molecular weight heparin has a rapid redistribution phase followed by an
unsaturatable first order elimination phase t1/2life ~4hours. This dose
independent clearance and longer half life is due to the fact that the low molecular
weight heparins are less bound to macrophages
- Note that the half life of unfractionated heparin in the controlled clinical situation
can be assumed to be 0.5 - 1hour. With very high doses, the apparent half life may be
vastly increased.
Excretion
- Hepatic clearance predominates for the unfractionated heparin
- Low molecular weight heparins have a renal clearance that is slower than their hepatic
clearance, reflecting a decreased binding to macrophages. Laboratory monitoring is
mandatory in renal insufficiency.
Chemistry
Heparins are strongly negativly charged, acidic glycoaminoglycans
(mucopolysaccharides) consisting of chains of alternating residues of D-glucosamine and
uronic acid, either glucuronic acid or iduronic acid.. Unfractionated heparin
consists of chain lengths which vary from 3000 to 30 000 Daltons.
The low molecular weight preparation consists of chain lengths which vary from
3000 to 9000 Daltons. They are manufactured from unfractionated porcine heparin by
controlled depolymerisation
- Chemical - Nitrous oxide, alkaline hydrolysis or peroxidative cleavage..
- Enzymatic - Hepariniase
Dose
| Indication |
Fixed Dose Unfractionated Heparin |
Adjusted Dose Unfractionated Heparin |
Fixed Dose
Low molecular weight heparin |
DVT PROPHYLAXIS
General Surgery
Medical conditions |
5 000U sc 8-12 hourly |
Adjusted sc to aPTT upper range of normal |
Dalteparin 2 500U sc daily
Enoxaparin 2 000U sc daily |
DVT PROPHYLAXIS
Orthopaedic Surgery
Acute Spinal Injury
Multiple Trauma |
Considered inadequate |
Adjusted sc to aPTT upper range of normal |
Dalteparin 5 000U sc daily
Enoxaparin 3 000U sc daily |
DVT TREATMENT |
Considered inadequate |
Infusion to
aPTT 1.5-2.0 normal
= heparin level of 0.2-0.4U/ml |
Dalteparin 100U/kg twice daily
Enoxaparin 100U/kg twice daily
antiXa levels of 0.1-0.2 U/ml |
Acute myocardial ischamia
Acute vascular insufficiency
Haemodialysis |
Considered inadequate |
Infusion to
aPTT 1.5-2.0 normal |
Dalteparin 100U/kg twice daily
Enoxaparin 100U/kg twice daily |
| Cardiopulmonary bypass |
Considered inadequate |
Infusion to
aPTT 2.0-3.0 normal |
No data available |
Effects
- Antithrombin, anti-Xa and extrinsic pathway inhibitor effect
- Anticoagulation and prevention of clot propagation
- Haemorrhage - local at the site of injection and systemic especially GIT
- Thrombocytopaenia
- Mild nonidiosyncratic reaction - heparin binds to platelet factor 4 causing
platelet activation. Occurs on 2-4 day of treatment with unfractionated heparin and
resolves spontaneously during continued therapy
Low molecular weight heparin binds less avidly to platelet factor 4 and is less
likely to cause thrombocytopaenia.
- Severe IgG or IgM mediated "auto immune" thrombocytopaenia directed
against heparin bound to platelet factor 4.
This forms a complex on the surface of the platelet and activates the Fc receptor
causing widespread platelet degranulation.
The antibody cross reacts with the endothelial cell surface glycosaminoglycans bound
to platelet factor 4 causing severe antibody mediated vascular injury with thrombus
formation.
The syndrome occurs 6-7 days after initial exposure, predominantly to unfractionated
heparin and will only terminate with withdrawl of heparin. Re-exposure to heparin,
even low molecular weight heparin will re-establish the heparin induced thrombocytopaenia.
- Alopecia
- Hypoaldosteronism with associated metabolic acidosis and hyperkalaemia
- Osteoporosis related to 20 000 units / day for 6 months especially in pregnancy
- Reduction in plasma triglycerides due to release of lipoprotein lipase
- Regulation of angiogenesis and maintenance of endothelial wall competence
- Antiproliferative effect on vascular smooth muscle after endothelial injury
Formulation
Heparin is currently obtained from the lung or gut mucosa of cattle and pigs.
It is available as either a sodium or a calcium salt.
- Unfractionated Heparin injection
- 1 000 units/ml
- 5 000 units/ml
- Unfractionated heparin as Calciparine
- 5000 units/syringe of 0.2ml = 25 000 U/ml
- Low molecular weight heparin
- enoxaparin - Clexane 20mg/syringe of 0.2ml = 100mg/ml
- dalteparin - Fragmin
Inadequate heparin response
- Specific disease states
- Antithrombin III deficiency
- Hypereosinophilia
- Coronary artery disease
- Drug interactions
- Previous heparin therapy
- Oral contraceptives
- Errors of administration
- Wrong product
- Wrong dose
- Wrong route
- Others
- Ongoing active coagulation
- Advanced age.
Reversal
- Allowing spontaneous dissipation of heparin effects
- Protamine sulphate - acid/base, anionic/cationic reaction forms a harmless
precipitate. Protamine has intrinsic anticoagulation properties of its own and must be
titrated. 1.3mg/Kg/100 units unfractionated heparin still predicted to be
circulating. Protamine is available as 10mg per 5ml
- Adverse effects
- Hypotension -
The Heparin/Protamine complex releases histamine, predominantly from lung
macrophages. This can be decreases by giving protamine slowly and into the arterial
circulation allowing dilution of the complex prior to exposure to the lung
- Pulmonary hypertension -
The Heparin/Protamine complex activates complement and causes thromboxane release.
Pre-treatment with a cyclooxygenase inhibitor is said to help
- Allergic reactions
- True mediated by antibodies formed on prior exposure to protamine
- Previous bypass surgery
- Protamine containing insulins
- Fish allergy
- ??? vasectomised males
- Immediate mediated by complement
- Protamine sulphate blocks bleeding induced by low molecular weight heparins in
laboratory animals, but there have been no studies in humans.
- Alternative cationic alkaline agent
- Recombinant platelet factor 4 (2.5 to 5mg/kg)
- Hexadimethrine - hypotension, pulmonary hypertension and anticoagulation in
excess
- not a popular drug!
- Toludine blue
Heparin Protocol
(This protocol is only a guideline. Many other protocols have been
proposed.
It is essential that it is applied in the context of sound clinical judgement!)
- Stop Warfarin 3 days prior to surgery
- INR must be less than 1.5 for surgery
- Intravenous low dose Vitamin K (phytomedine) e.g. 0.5-1.0mg [ Shetty et al. Haemostas
1992 67 pp53-62 ] and / or Fresh Frozen Plasma may be used with caution, if urgent surgery
is required.
- Start Heparin infusion and adjust according to the aPTT or ACT
- Stop the heparin infusion 3 hours prior to surgery
- Meticulous intra-operative haemostasis is required
- Restart the heparin infusion 12 hours after the surgery
- Neurosurgery - Restart the heparin infusion 24 hours after the surgery
- Ophthalmology - Consult with the surgeon involved
- Restart the warfarin as soon as oral medication is possible
- Stop the heparin when the INR is adequate
- Baseline full blood count, platelet count, INR and aPTT/ACT
- Bolus with 80 Units/Kg of heparin
- Start heparin infusion at 18 Units/Kg/hr
- 20 000 Units heparin per 200mls Normal Saline = 100 Units/ml
- (Units/Kg/hr x Weight)/100 = mls/hr for infusion
- Check coagulation profile regularly
- aPTT must be checked
on a daily basis
- aPTT must be checked
six hours after every heparin dose adjustment
- ACT must be checked on an hourly
basis
- Platelet count must be checked every three days
| ACT |
aPTT |
Response |
| > or = Pt's Normal |
< 35 |
Bolus with 80 Units/Kg
Increase Infusion by 4 Units/Kg/hr |
| < 1.5x Pt's Normal |
36-45 |
Bolus with 40 Units/Kg
Increase Infusion by 2 Units/Kg/hr |
| 1.5-1.9 x Pt's Normal |
46-70 |
No Change |
| 2-2.5 x Pt's Normal |
71-90 |
Decrease Infusion by 2 Units/Kg/hr |
| >2.5x Pt's Normal |
>90 |
Stop Infusion for 1 hour
Restart Infusion at a rate reduced by 3 Units/Kg/hr |
Action
Blockage of vitamin K reductase and vitamin K epoxide reductase:
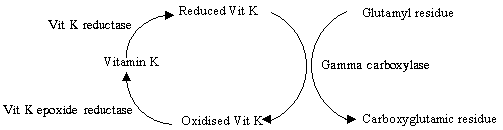
The inhibition of the gamma carboxylase leads to an accumulation of the inactive
precursors of the clotting factors - also known as protein induced by vitamin K absence -
PIVKA. This affects factors II, VII, IX, X, Protein C, Protein S - the serine protease
enzymes
Biokinetics
Absorption
- Rapid and complete oral absorption
Distribution
- Confined to the extracellular space - 200ml/Kg
- Extensive binding to albumin
- Peak plasma level seen 2-6 hours with a terminal half life of ~40 hours.
- Anticoagulation effect first seen in 8-12hours (Elimination half life of previously
formed factors).
- Maximum anticoagulation in 36-72 hours (2-3 days)
Metabolism
- Liver to inactive metabolites
Excretion
- Liver with biliary excretion, intestinal deconjugation and reabsorbtion.
Chemistry
Derivative of 4-hydroxycoumarin.
Dose
Initial bolus dose 0.2mg/kg to a maximum of 10mg, given daily for 2 days
Adjusted daily dose titrated against factor activity, measured by the prothrombin
time / INR for factor VII activity.
- The American College of Chest Physicians Guidelines
- Chronic Atrial fibrillation: INR 2.0 to 3.0
- Prevention of deep vein thrombosis propagation: INR2.0 to 3.0
- Prevention of thrombosis on mechanical heart valves: INR 2.5 to 3.5
- Recent European investigations14 - Select a target INR within
the range
- Prevention of thrombosis on mechanical heart valves:
- Caged ball and tilting disk valves : INR 4.0 to 5.0
- Bileaflet valves INR 2.5 to 3.0
Warfarin Guidelines |
| INR |
Action |
| 1.1-1.4 |
Increase dose by 20% |
| 1.5-1.9 |
Increase dose by 10% |
| 2.0-3.0 |
Hold dosage |
| 3.1-4.0 |
Decrease dose by 10% |
| 4.1-4.5 |
Decrease dose by 20% |
| 4.6-5.0 |
Omit one dose
Restart dose at 20% less |
| >5.0 |
Omit until INR <4.5
Restart dose at 20% less |
Effects
- Inhibition of the formation of active factors II, VII, IX and X
- Anticoagulation
- Deep vein thrombosis prophylaxis
- Thromboembolic prevention in mitral valvular lesions and dilated cardiomyopathy
- Prosthetic valvular anticoagulation
- Atrial fibrillation associated thromboemboli prophylaxis
- Reduction in the thromboembolic events in acute myocardial infarction
- Haemorrhage
- Cranial, ears, nose, urinary tract, skin, adrenals, gastrointestinal tract
- Inhibition of the active forms of Protein C and its cofactor Protein S
- Transfer across the placenta
- Embryopathy
- Teratogenicity - nasal hypoplasia, stippled epiphyses, blindness and frontal
bossing
{like the Conradi-Hunnerman type of chondrodysplasia punctata!}
Formulation
Coumadin 5mg tablets, Warfarin 3 and 5mg tablets
Interactions
- Protein binding displacement
- Phenytoin, nalidixic acid, oestrogens, metronidazole, miconazole
- Depletion of intestinal vitamin K sources
- Cephalosporins, cefamandole, moxalactam
- Small intestine disease
- Impaired vitamin K synthesis
- Anabolic steroids
- Inadequate diet
- Enzyme induction with increased metabolism
- Barbiturates, rifampicin, griseofulvin, nafcillin, inhalational anaesthetics
- Increases in factors VII, VIII, IX and X
- Pregnancy
- Autosomal dominant trait
Clinical risk factors - European Consensus Group Summary
- Low Risk
- Minor surgery, less than 30 minutes duration
- < 40 Years old with no additional risk factors
- Moderate Risk
- Major surgery, longer than 30 minutes duration
- Virchow's Classic Triad
- Local Trauma
- Statis
- Hypercoagulability
- > 40 Years old
- Oral contraceptive medication within the last six weeks
- Immobilised medical patients with active disease
- Body mass index >30
- Heavy cigarette smoking
- High blood pressure
- High Risk
- Previous deep vein thrombosis
- Major surgery for malignant disease
- Orthopaedic surgery to the lower limbs
- Systemic Immune Response Syndrome
- Stroke, congestive cardiac failure and acute myocardial infarction.
- Thrombophilia
Diagnosis
Diagnostic Algorithm for deep vein thrombosis -
Compression ultrasound with or without doppler flow assessment is the optimum non invasive
test for symptomatic patients
Pulmonary embolism
- For patients with symptoms of deep vein thrombosis and pulmonary embolism the
Diagnostic Algorithm should also be followed
- Patients with normal chest radiographs should be investigated with a ventilation -
perfusion scintigram
- Patients with radiographic abnormalities, likely to cause an indeterminate
ventilation - perfusion scan, should have spiral computed tomography of the
pulmonary vessels, if available!
Prevention
- Physical methods
- Graduated elastic stockings
- Arterial-venous impulse foot pump
- Mobilisation
- Pharmacological methods
Deep Vein Thrombosis
Prevention and treatment |
Abbreviations: LMWH = Low Molecular Weight Heparin, UFH = Unfractionated heparin.
|
| CONDITION |
Fixed Dose
UFH |
Adjusted Dose
UFH |
Fixed Dose
LMWH |
Warfarin |
 |
 |
 |
 |
|
DVT PROPHYLAXIS
General Surgery
Medical conditions |
5 000U sc 8-12 hourly |
Adjusted sc to aPTT upper range of normal |
Dalteparin 2 500U sc daily
Enoxaparin 2 000U sc daily |
Considered unneccessary |
DVT PROPHYLAXIS
Orthopaedic Surgery
Acute Spinal Injury
Multiple Trauma |
Considered inadequate |
Adjusted sc to aPTT upper range of normal |
Dalteparin 5 000U sc daily
Enoxaparin 3 000U sc daily |
Adjusted oral dose to INR 1.5-2.0 |
DVT TREATMENT |
Considered inadequate |
Infusion to
aPTT 1.5-2.0 normal
= heparin level of 0.2-0.4U/ml |
Dalteparin 100U/kg twice daily
Enoxaparin 100U/kg twice daily
antiXa levels of 0.1-0.2 U/ml |
Adjusted oral dose to INR 2.0-3.0 |
Acute myocardial ischamia
Acute vascular insufficiency
Haemodialysis |
Considered inadequate |
Infusion to
aPTT 1.5-2.0 normal |
Dalteparin 100U/kg twice daily
Enoxaparin 100U/kg twice daily |
Adjusted oral dose to INR 2.0-3.0 |
| Cardiopulmonary bypass |
Considered inadequate |
Infusion to
aPTT 2.0-3.0 normal |
No data available |
To long onset and neutralisation |
Surgical specialities
- Urology
Mechanical methods are preferred as haemostasis is difficult during endoscopic
procedures
- Head and neck surgery
Mechanical methods are preferred as the vascularity of the area makes haemostatis
difficult
- Neurosurgery
Mechanical methods are preferred as the consequences of an intracranial bleed are so
severe.
A Systemic thrombohaemorrhagic disorder seen in association will a well defined
clinical situations, with laboratory evidence of procoagulant activity, fibrinolytic
activation, inhibitor consumption and biochemical evidence of end organ damage or failure.
Clinical diagnosis
Clinical evidence of
- Microvascular Thrombosis manifests as Organ system dysfunction. The small and
large vessel thrombosis with impairment of regional blood folw, ischaemia and end organ
damage causes the Morbidity and Mortality
- Haemorrhage manifests as petechiae, purpura, gangrene and abnormal bleeding from
wounds and intravascular access sites
occuring in the appropriate clinical setting
- Cardiovascular diseases
- Transfusion reactions, massive transfusions and haemolysis
- Indwelling Prosthetic material eg. Intra Aortic Balloon Pump
- Haematological malignancy - Acute myelocytic leukaemia and Acute promyelocytic
leukaemia
- Vasospastic Disorders
- Diabetic angiopathy
- Autoimmune angiopathy -Leriche syndrome in rheumatoid arthritis, systemic lupus
erythematosis, scleroderma and sjorgens syndrome
- Raynauds syndrome
- Obstetric
- Abruptio placentae
- Abortions
- Amniotic fluid embolus
- Eclampsia
- Intra-uterine death
- Infections
- Bacteria
- Gram negative lipopolysaccharide
- Gram positive mucopolysaccharide
- Viral
- Human Immunodeficiency Virus
- Cytomegalovirus
- Varicella Zoster Virus
- Hepatitis B Virus
- Injury
- Burns
- Crush Syndrome
- Ischaemia to peripheries
- Massive trauma
- Severe acidosis and alkalosis
- Liver disease
- Acute hepatic failure
- Obstructive jaundice
Pathophysiology
The common end point in all of the precipitating conditions is an uncontrolled
circulation of phospholipid in the blood, disrupting the normal localisation of the
clotting process and allowing plasmin and thrombin to circulate freely. These
proteolytic enzymes set up a circular pathophysiology of
- Procoagulant system activation
- Fibrinolytic system activation
- Inhibitor consumption
- Cytokine release
- Cellular activation
- End organ damage
- Thrombin causes substantial microvascular and macrovascular thrombosis
- Initiation of the procoagulation pathway
- Direct production and stabilisation of fibrin
- Release of Platelet Activating Factor
- Release of monocyte tissue factor
- Endothelial damage and destruction
- Tumour Necrosis Factor, Interleukin 1and Interleukin 6 cause direct damage
- Endothelin causes intense vasoconstriction and vasospasm
- Factor XII activation of the kinin system
- Enhances the fibrinolytic pathway
- Release of thrombomodulin
- Plasmin is responsible for the haemorrhage
- Fibrinolysis
- Release of plaminogen activator 1
- Inhibition of the procoagulation pathway
- Cleaves Fibrinogen to form Fibrinogen Degradation Products X, Y, D and E and
degrades cross linked fibrin monomers in D-Dimers
- FDPs interfere with fibrin monomer polymerisation impairing haemostatis
- Biodegrades factors V, VIII, IX, XI and other plasma proteins.
- ENhances the procoagulation pathway
- FDPs induce monocyte release of tissue factor enhancing thrombosis
- Activates the complement cascade
- Causing red cell and platelet lysis, releasing more procoagulant material.
- Increasing vascular permiability with hypotension
Laboratory Tests
- Procoagulation activity
- Increased Prothrombin fragments 1 and 2
- Increased fibrinopeptide A
- Increased fibrinopeptide B
- Increased Thrombin AntiThrombin complexes (TAT)
- Increased D-Dimer
- Fibrinolytic activity
- Increased D-Dimer
- Increased Fibrin Degradation Products
- Increased Plasmin
- Increased Plasmin AntiPlasmin complexes (PAP)
- Inhibitor consumption
- Decreased Antithrombin III
- Decreased alpha 2 Antiplasmin
- Decreased Heparin CoFactor II
- Decreased Protein C or S
- Increased Thrombin AntiThrombin complexes (TAT)
- Increased Plasmin AntiPlasmin complexes (PAP)
- End organ dysfunction
- Decreased pH
- Decreased PaO2
- Increased Creatinine
- Increased Lactate Dehydrogenase
Marker |
DIC |
Primary lysis |
Thrombotic Thrombocytopaenic Purpura |
| Prothrombin Fragment 1+2 |
Elevated |
Normal |
Normal |
| D-Dimer |
Elevated |
Normal |
Normal/Elevated |
| Antithrombin III |
Decreased |
Normal |
Normal |
| Fibrinopeptide A+B |
Elevated |
Normal |
Normal |
| Platelet Factor 4 |
Elevated |
Normal |
Elevated |
| beta-Thromboglobulin |
Elevated |
Normal |
Elevated |
| B-beta 15-42 Peptide |
Elevated |
Normal |
Normal |
Treatment
- Elimination of the cause of phospholipid release
- Replacement of Antithrombin III (Personal
viewpoint - Limited published data)
- Antithrombin III is consumed in the development of disseminated intravascular
coagulation
- The deficiency of Antithrombin III as a multipotent inhibitor is pathological,
exacerbating the process of disseminated intravascular coagulation
- Patients who have AT-III levels under 50% are gravely ill. Such AT-III levels are
strongly predictive of death in patients with DIC.
- Replacement of Antithrombin III in patients with markedly diminished activity is of
therapeutic benefit, rapidly "turning off" the DIC, and may in fact diminish
mortality.
- Replacement of other inhibitors
- Protein C and S
- Heparin Cofactor II
- alpha 2 Antiplasmin
- Anticytokine agents
- Administration of heparin is controversial, especially as this dramatically shortens the
half-life of AT-III
- Administration of procoagulation factors is very controversial. If specific factor
deficiencies have been identified, such replacement just may be of benefit.
The role of platelet transfusions, especially those given prophylactically to
forestall bleeding remains controversial. The currently accepted threshold for
platelet transfusion was derived from a 1962 study by Gaydos. In this study patients
with leukaemia who had a platelet count of less than 20x109, had an increased
frequency of gross haemorrhage. The study did not try to establish a threshold value
that prevented all bleeding. There are a number of problems with giving a platelet
transfusion that must be considered prior to the indiscriminate usage of this blood
product.
- Collection
- Random donor platelets are centrifuged from a freshly collected unit of blood. It
consists of:
Platelets (average 7x1010) - 4-10 units are combined to have an adequate
therapeutic effect.
Numerous contaminating white cells - Sensitise the hosts immune system against the
human leukocyte antigen system. Leukocyte depletion techniques are gaining in
popularity.
Plasma.
- Single donor platelets are collected by apheresis from a freshly collected unit of
blood. It contains 5x1011 platelets all with the same antigenic type.
- Storage
- Platelets must be stored at room temperature and constantly agitated to facilitate
gas exchange, otherwise they lose their ability to aggregate
- Bacterial growth is promoted by this method of storage, limiting the storage time
to five days.
- Compatibility
- Antibodies from prior exposure to alien white blood cells and platelets can destroy
new platelets by attacking:
Class I HLA proteins (platelets only have class I HLA proteins and so do not
effect primary immunisation)
ABO proteins
Platelet-specific proteins
- There is no reliable compatibility test for screening platelet components.
- Transfusion "triggers"
- Surgical bleeding may become excessive at platelets counts less than 75x1010/L
- Spontaneous small vessel bleeding does not increase until the platelet count is
less than 5x1010
[ Rubella, P. The Threshold for Prophylactic Platelet Transfusions in Adults with
Acute Myeloid Leukaemia N Engl J Med 1997 337 pp1870-1875 ]
The risk of major bleeding is similar with platelet transfusion thresholds of 20x1010/L
and 10x1010/L when there is active bleeding or invasive procedures were needed.
- Transfusion "failures"
- The number and condition of stored platelets are often suboptimal, with early
demise of the transfused platelet
- Fever, splenomegaly, disseminated intravascular coagulation and
drugs in the recipient rapidly destroy transfused platelets
- Alloimmunisation increases logarithmically with the number of platelet transfusion.
Bibliography

