Back Up
List of Abbreviations | |
| pH i | Gastric intramucosal pH |
| TEE | Trans-(o)esophageal echocardiography |
| CVP | Central Venous Pressure |
| PCWP | Pulmonary Capillary Wedge Pressure |
| NO | Nitric oxide |
| DO 2 | oxygen delivery |
| VO 2 | oxygen consumption |
| Shv O2 | Hepatic venous oxygen saturation |
| A note for Americans. We still favour the word adrenaline instead of "epinephrine", and use noradrenaline for norepinephrine. Get used to them - and, yes, we know that the international trend is towards using the word epinephrine. Do we care? | |
Inotropes are used to support the failing heart. The heart may fail in several circumstances, and a variety of drugs may be used to support it. Sometimes we encounter difficulty in distinguishing between drug properties that support the heart and those that affect the peripheral circulation. All in all, a vast fog of confusion overshadows the everyday use and abuse of inotropes. We will try and dispel this fog, and engender in the mind of the reader a practical approach to inotropic support. To do this, we first need to understand some basic physiology.
The circulation exists to provide the tissues with oxygen and nutrients, and to remove waste products. Over hundreds of millions of years, an ingenious system has evolved - the cardiovascular system maintains a head of pressure, and each organ diverts flow to serve its needs. If the pressure decreases somewhat, then two things happen:
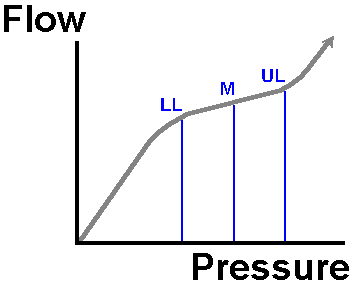
Initially, if the mean pressure in the vessels perfusing a tissue drops, the vessels in the tissue dilate. This vasodilatation preserves blood flow to the tissue, and its metabolic demands are still met. Down to a pressure of about 75% of normal, this miraculous autoregulation still preserves tissue flow. Below this point, tissue perfusion drops off progressively. This property of most tissues in the body is shown in the following diagram.

Note how flow changes little when we vary pressure from the normal level (N) down to the lower autoregulatory threshold (LL) or indeed the upper limit (UL). Below or above these limits, the tissue fails to autoregulate. This concept of tissue autoregulation is vital.
Even below a pressure of 75% of normal, the tissue tries its best to preserve its metabolic functions. It does this by extracting more nutrients and (especially) oxygen from that small quantity of blood still reaching it, and excreting wastes into this blood as it passes through. Only at very low levels of tissue perfusion, does the tissue "give up", and resort to anaerobic metabolism, with a drop in tissue pH, and production of lactate.
We know that blood pressure depends on cardiac output and the resistance of the vessels into which the heart is pumping. A drop in either will decrease the mean pressure in the system. A variety of intravascular sensors exists to sense such a drop. We can see that the body might compensate for a pressure drop in two ways - it might increase the cardiac output, or it might increase the total peripheral resistance of the system. In fact, when faced with a drop in pressure, compensatory mechanisms exist to both raise peripheral resistance and increase cardiac output. These short-term mechanisms are mainly mediated by the sympathetic nervous system.
You can also deduce that a substantial drop in pressure is ominous: It signifies one or more of the following:
But these are not the only patients we see who are afflicted by failure of their cardiovascular system to provide sufficient pressure to perfuse their organs. There are others. They include a vast number of patients who have a definable, reversible cause for their hypotension. Broad groups include:
|
Remember: Always look for a reversible cause for hypotension.
Ask: "What is the cause?" |
We will in turn look at the various causes of hypotension, and the role of inotropes in each of them. But first, a word of caution. If you take a dog and acutely remove sufficient blood from its circulation to drop mean arterial pressure to say forty percent of normal, and then .. wait .. and then re-infuse the blood after say three hours, after an initial recovery, the dog will die. Despite return of its blood, mechanisms will be set in place that put it on the road to irreversible cardiovascular collapse. It is this that we wish to avoid in our patients, and it is immediately obvious that the following are all important:
In any hypotensive patient, we should be able to establish a short list of achievable goals. The following short list seem reasonable:
Unfortunately, here we have a problem. What is "adequate fluid resuscitation"? My short answer is that nobody knows! Let's look at a few possible criteria:
What a mess! Each of the above has its failings. Central venous pressure may for example be low in someone with gross overfilling of the left side of the heart, if they have left-sided heart failure. We know that there is extremely poor correlation between pulmonary capillary wedge pressure and good measures of left-sided filling such as echocardiography. [See for example Jardin F et al, Int Care Med 1994 20 550-4 for an example of how really bad the correlation is] "Metabolic parameters" too have their up and down-sides. And, to make us even more miserable, our assessment of function in vital organs such as the brain, kidney, liver, bowel and heart is often sorely lacking.
You will also immediately note that there is a major problem in the above - if we are waiting for our fluid resuscitation to become "adequate", might we not miss the boat, and fail to start inotropes timeously? Remember our dog? The real questions then are:
| 1. When do we cut back on our fluid resuscitation? |
| 2. When do we start inotropes? |
I know of no definitive answer to the above. My partial answer would be:
| 1. Aim for a reasonable CVP, or if you have the expense and luxury of a Swan-Ganz, a reasonable PCWP; or better still, adequate filling on echocardiogram. Some practical suggestions occur right at the end of this web page. |
| 2. Start inotropes SOON, as discussed below. |
We know that in the normal organism, a whole host of homeostatic mechanisms exist to maintain the head of pressure perfusing the tissues. If systemic pressure drops below a certain level, then tissues will receive insufficient flow, and tissue autoregulatory failure will follow. The body will move heaven and earth to prevent this state, and so a substantially lowered arterial pressure represents failure of each and every homeostatic mechanism.
We therefore need to know the individual autoregulatory threshold for that organism before we can apply any sort of rule. If we know a person's pre-morbid blood pressure then we can confidently predict that reduction in mean pressure of over about 30% will result in a substantial decrease in perfusion of vital organs such as the brain. Lower values will cause gross dysfunction! It is not simply enough to assume that the blood pressure was say 120/80, giving a mean of about 93 mmHg. In hypertensives, the curve is shifted to the right. This has been well shown in the brain [Conway J, Physiol Rev 1984 64.2 pp617-57; Lassen NA, Phys Rev 1959 39 pp183-238; Finnerty FA et al, J Cl Invest 1954 33 pp1227-32 ] where dropping the pressure to within "the normal range" may result in hypoperfusion.
This immediately gives the lie to studies that arbitrarily choose a "target" mean arterial pressure as their resuscitative goal (many studies in the literature) without reference to the pre-morbid mean arterial pressure of the subject. Even if you previously recorded just one blood pressure on that patient, and the value was 140/90, (giving a mean arterial pressure of 90 + (140-90)/3 = 107 mmHg), it is clearly unwise to be satisfied with a mean arterial pressure of say 75 mmHg as your end point, with vital organs poised on the brink of autoregulatory failure.
This also suggests that if we have absolutely no idea of what the patient's "normal" blood pressure runs at, we should be generous in our estimates, especially if the person comes from a population or age group where hypertension is prevalent, or there is other evidence of hypertension, such as funduscopic changes, or left ventricular hypertrophy.
To me this is a convincing argument that, in the absence of evidence to the contrary (prior lowish blood pressures, no history of hypertension, normal funduscopy, etc) we should perhaps ..
| Aim for the magic target of: a mean arterial pressure of 100 mmHg! (But have an open mind, and reassess this goal in the light of
|
Why do I choose 100mmHg? This allows us to cover a fairly substantial range of pressures, and is often a realisable goal. Think about it - whether the patient normally has a mean arterial pressure of 70mmHg (say a blood pressure of 90/60) or one of 120mmHg, we will probably still be keeping their pressure within the autoregulatory range of their vital organs! Note however that although lowering the pressure below the autoregulatory threshold almost certainly implies inadequate perfusion, the converse is not necessarily true - good pressure need not imply adequate perfusion!
Remember too that the autoregulatory threshold of 70% of the usual mean is for normals - unfortunately, similar figures do not exist for the critically ill. If anything, we should assume that in such patients tissue autoregulation is less rather than more exact, with a consequent narrower range of tolerance for low pressures.
A short list of criteria for assessing organ function could include the following:
This seems to be the most clear indication for the use of inotropic support. If the cardiac pump has failed, then surely the correct approach is to give something that will stimulate contractility? There are however two situations where this pump fails:
We are therefore left with the situation of acute heart failure. Here it would seem reasonable to administer inotropes, at least until that uneasy transition zone where we lapse into chronic failure (Where we know that inotropy is harmful overall) - the only problem being that nobody can tell us when this transition from supposed benefit to near- certain harm occurs! There also seems to be little consensus on what we should give. The general feeling in acute failure following on acute myocardial infarction seems to be that agents with predominant beta-agonism are desirable. The metabolic evidence is all on the side of advocates of this approach, as agents such as dobutamine appear to have a favourable side-effect profile, and seem to be minimally damaging to the myocardium. Adrenaline, in contrast has been implicated in extension of myocardial infarct size and would therefore appear to be contra-indicated.
But wait a bit! Consider the patient that quite literally drops dead in the street. What does the evidence say there? Quite unequivocally, we read that in the acute resuscitative situation, adrenaline is the drug! Even worse, agents such as isoprenaline {isoproterenol} with predominant beta effect are almost certainly harmful. This presents us with a rather unpleasant dilemma - if you collapse in the street, we know you will benefit from large doses of adrenaline, but, god help you, if you collapse somewhat less dramatically in the coronary care unit, probably the last agent you will receive is that very same adrenaline. (Perhaps I'm being a little unfair, but this statement should at least cause momentary concern in those who reflexly administer agents with predominant beta effect to their acute cardiac patients). Strangely enough it is also the beta receptor that up-regulates in ischaemic heart muscle, and is thought to cause problems!
These contrasting pictures outline what I consider to be the truth - that some, less ill "coronary" patients may well benefit from beta agonism, while the sicker, more acute patients may actually be harmed by the very same agents, and these unfortunates probably need inotropes with alpha effect! Again, a knee-jerk approach to therapy is the approach of a jerk, and certainly not that of the thinking physician.
| Summary: Problems with treating the failing pump | |
| 1 | It seems unwise to lash the chronically failing heart |
| 2 | When does "acute" become "chronic"? |
| 3 | When are alpha agonists (a) vital and (b) deleterious? |
A variety of conditions exist where there appears to be "failure of the peripheral vasculature", with or without myocardial depression. This failure is common:
| The primary treatment of both haemorrhagic and septic shock is fluid repletion |
This is not to say that fluid replacement is always sufficient therapy, but often in the hurly burly of fighting over "which inotrope is best", we tend to forget that without something to pump, a pump will not work. All studies that use inotropes should first be assessed as to whether their primary goal was adequate fluid resuscitation. If this primary goal was not met, then all subsequent conclusions are meaningless.
Where gross and excessive peripheral vasodilatation is present (as is almost always the case in septic shock) it seems entirely reasonable to administer agents that antagonise this vasodilatation. Where there is evidence of (or perhaps even a suspicion of) impaired heart performance, addition of an agent that augments such performance seems wise. We will defer discussion of actual agents until later.
Discussing normal tissue regulation, we noted that tissues have to be severely underperfused before they limit their metabolic activity and seek energy sources other than aerobic metabolism. One might suspect that this holds good for septic and other critically ill patients, in other words that oxygen consumption should diminish little with declining oxygen delivery, until a very low value of delivery is reached.
Unfortunately, some disagree. For example, Danek et al. found that in some critically ill patients there appeared to be co-variation of supply and oxygen consumption. [Am Rev Respir Dis 1980 122 pp387-95 ] This has been termed "pathological O 2 supply dependency". The implication of Danek's and many subsequent studies is that supply may be inadequate in some critically ill patients, even if oxygen delivery appears to be in the normal range, and that consequently such patients have a metabolic demand that is not being fulfilled. A hypothesis has been tagged onto this - that increasing supply will improve tissue function and decrease morbidity and mortality. This has two practical implications:
Neither of the above has held up well to scrutiny.
For some years now, it has been postulated that certain critically ill patients benefit from aggressive inotropic support. Since the seminal article of Shoemaker et al appeared, claiming the benefits of using of a Swan- Ganz catheter to enhance cardiac output above mere "resuscitative" levels, others have endorsed (or occasionally even tried to duplicate) this approach, with varying degrees of failure. Currently, people seem to be shying away from "supranormalisation", based on the paucity of evidence that it works, [ Gattinoni L et al, NEJM 1995 333 1025-32 ] and some articles that suggest it may even increase morbidity and mortality [Hayes et al, N Engl J Med 1994 330 1717-1722 ].
The dream of increasing supply to the point where demand suddenly levels off does not unfortunately appear to have a strong base in reality! There are several possible reasons for this:
Vincent & De Backer have reviewed the concept of supply dependency [Acta Anaesthesiol Scand 1995 39 S107 pp229-37 ] and, despite some rather tortuous arguments, conclude probably correctly that "instead of aiming at a given level of supranormal DO2 in all patients, it is probably more desirable to tailor therapy according to the needs of the patient at any given time, based on an assessment of organ system function."
| Summary: Problems with supranormalisation | |
| 1 | Little evidence of benefit, concerns about risk |
| 2 | Poor standardisation of methodology |
| 3 | Concerns about unnecessarily beating the failing organism |
It can be seen that in our use of catecholamines as inotropes, we are limited to a relatively small selection of receptors that we can stimulate. The main factor difference then between the various inotropes is their differing potency and efficacy at various receptor types:
| Receptor Stimulation by various Catecholamines | ||||||
| AGENT | Alpha 1 | Alpha 2 | Beta 1 | Beta 2 | Beta 3 | Dopaminergic |
| Adrenaline (epinephrine) |
+++ | +++ | ++ | ++ | ++ | - |
| Noradrenaline (norepinephrine) |
++ | ++ | ++ | - | +++ | - |
| Dobutamine | +- | - | +++ | + | ? | - |
| Dopamine | ++ | ++ | ++ | + | ? | +++ |
| Dopexamine | - | - | + | +++ | ? | ++ |
| Isoprenaline (isoproterenol) |
- | +- | +++ | +++ | +++ | - |
| Ephedrine | + | ? | ++ | ++ | ? | - |
| Phenylephrine | +++ | ? | - | - | - | - |
The above table is extremely simplistic, especially as there are three subtypes of the alpha-1 and alpha-2 receptors. There are at least two dopaminergic receptors (DA1 and DA2). Ephedrine also has an indirect action, depending on noradrenaline release for some of its effects. Dobutamine is a racemic mixture: the (-) isomer is a potent alpha-1 agonist and is ten times more potent as a beta agonist than is the (+) enantiomer which is also a potent alpha-1 blocker!! (See Hardman 1996). Source texts differ slightly concerning the effects of various agents at various receptors, and ideally the above table should contain receptor affinities and tissue responses for a variety of different tissues. The table is thus almost useless in its current form.
A wealth of controversy has developed over the competing merits of the various agents. This has been fuelled by the prices of the agents - adrenaline and noradrenaline are cheap, while agents such as dobutamine are rather pricey.
Based on the above, we can identify three broad groups of agents:
A simplistic glance would lead us to believe that where the heart is failing, and the peripheral vasculature appears to be in good order, an agent with predominant beta effect (especially a beta-1 selective inotrope) would be a good choice; where there is vasodilatation, perhaps an agent with alpha agonism is good, and with the combination of failing heart and dilated peripheral vasculature, we should either give an agent with mixed effect, or combine agents with alpha and beta effects. Not so! Controversy rages about this topic, perhaps more so than anywhere else in the field of intensive care medicine. Claims and counter-claims proliferate. Here is a brief (and biased) review of the agents.
Dobutamine
As mentioned above, this is considered by many a reasonable choice with moderate
degrees of myocardial dysfunction, especially in the presence of myocardial
ischaemia. Sometimes it fails outright, even in these circumstances.
Many have advocated its
use in septic shock - we can find little justification for this. Dobutamine
administered in severe septic shock, even up to doses of 20-30micrograms/kg/minute
frequently fails to meet realistic goals in terms of adequate perfusion
pressures, and this is not surprising in view of its minuscule of alpha agonist
effect in the face of the gross peripheral vasodilatation seen in such
patients. According to Leier, a
substantial part of dobutamine's central haemodynamic effects
may be mediated through peripheral vasodilatation !
Some have shown increases in DO 2 and VO 2 with dobutamine administration [Vincent JL et al, Crit Care Med 1990 18 pp689-93 ], and associated improvement in pH i [Silverman et al, Chest 1992 102 pp184-8; Gutierrez et al, Am J Resp Crit Care Med 1994 150 pp324-9 ] with for example 5 micrograms/kg/min of the same drug. Not everyone agrees, for example Schneider et al [Circ Shock 1987 23 pp93-106 ] showed that volume replacement alone restored splanchnic flow in septic pigs (with doubtamine adding nothing).
Isoprenaline
There seem to be few or no indications for use of this obsolescent
drug, with its nonspecific beta effects.
Dopexamine
Marvellous results have been claimed for this drug, including a 75%
reduction in mortality (22% to 6%) when administered pre-and post-operatively
in high-risk surgical patients. [Boyd O et al, JAMA 1993 270 pp2699-707 ]
This study involved an extremely mixed bag of patients, and some were admitted
pre-operatively, others only post-operatively. It is not clear why eight
patients in the control group had substantial post-operative haemorrhage
compared with one in the treatment group. Nevertheless, a study worth
reading.
Some researchers have claimed that "markers of visceral hypoperfusion" such as pHi may be improved by this agent. [See Smithies et al, Crit Care Med 1994 22 789-95; Maynard ND et al Chest 1995 108 pp1648-54 ] Others could not support this contention. [Uusaro A et al, B J Anaesth 1995 74 pp149-54 ] The status of dopexamine seems unclear at present, and we cannot recommend its use.
Dopamine
The enthusiasm of some groups for this agent appears almost boundless.
We cannot see why. Evidence for its efficacy is limited and contradictory.
It is also relatively expensive. In the past dopamine has been used for:
Adrenaline
This is our initial agent of choice in a variety of conditions, particularly
in septic shock. Many people would disagree with this choice, for a variety
of reasons. Some claim that splanchnic perfusion is impaired if adrenaline
is used. We would answer that in our opinion this is not the case
if the patients have been adequately resuscitated, but hard evidence is
lacking. In cases where the peripheral vascular resistance remains low
in the face of high doses of adrenaline (say, over 0.5 micrograms/kg/min),
we would add a pure alpha agonist such as phenylephrine to achieve adequate
vasoconstriction.
Antagonists of adrenaline use for septic shock include Meier-Hellman & Reinhart (1995), who have done a fair amount of work on hepatic venous oxygen saturation. They describe 'very preliminary' results (n=3) suggesting that adrenaline is bad news for Shv O2 , and have followed this up with a report (n=8) comparing adrenaline to dobutamine + noradrenaline, that also knocks adrenaline [Crit Care Med 1997 25 pp399-404 ], as do Levy et al [Intens Care Med 1997 23 pp282-7 ].
Noradrenaline
Use of this agent is still a vexing question - noradrenaline, has been
used to induce renal failure in animals, [Mills et al, Am J Physiol 1960 198 p1279 ]
and many authorities thus avoid it, although it has been used in extremis,
sometimes with startling success (even combined with dopamine)! Meier-Hellmann
& Reinhart (1995) describe various studies of noradrenaline which show conflicting and
indeed confusing results. Paul Marik, for example, showed improved pH i
with noradrenaline administration when compared with dopamine!
Meier-Hellman & Reinhart prefer the combination of noradrenaline + dobutamine
to adrenaline.
The effects of the various agents on regional perfusion may be extremely important. Experimental results obtained on animals or normal humans may not be relevant in the septic state. Inter-individual variation and pre-existing disease (for example hypertension) may also play a role. The modifying effect of septic shock on regional perfusion may also be complex:
Some studies have even shown that baseline splanchnic perfusion is increased in septic patients [Dahn MS et al, Surgery 1987 101 pp69-80 ; Johnson GA & McNamara JJ, Surg Forum 1981 32 pp24-6; Leevy CM et al, JAMA 1961 178 pp565-7 ] and endotoxin administration doubles splanchnic blood flow in normals [Fong Y et al, J Cl Invest 1990 85 1896-904 ]. Others suggest that the normal redistribution of blood away from the splanchnic circulation seen with a variety of inotropes does not occur in sepsis [Bersten AD et al, Surgery 1992 112 pp549-61 ], perhaps due to changed receptor sensitivity.
In contrast cerebral autoregulation appears to be preserved in patients with sepsis [Matta BF & Stow PJ, B J Anaesth 1996 76 790-4]].
Our traditional view that the inotropic properties of the heart are mainly dependent on beta-1 receptors is probably inaccurate. More evidence is emerging that there are beta-2 receptors in the myocardium, and that the large number of alpha adrenergic receptors there may also play an inotropic role. Also of interest is the rapid down-regulation that occurs with chronic stimulation of beta receptors - this does not appear to occur to the same degree with alpha receptors. An added concern is the role of the beta-3 receptor, which appears to (a) be present in the normal myocardium (b) to result in negative inotropy when stimulated, and (c) to lack the normal beta mechanisms for down regulation! [Gauthier C et al, J Clin Invest 1996 98 pp556-62, worth a read!]
Actual studies comparing the various inotropes have usually been performed on small numbers of patients, and have generally been contradictory. It may be that certain agents have more favourable effects on the perfusion of certain vital organs, and this may even translate into improved outcome in some patients, but the evidence supporting most assertions of this nature is very scanty. What should we then do?
| Summary: Which Inotrope? | |
| 1 | Nobody can say for sure! |
| 2 | Several bad & often conflicting studies do not help |
| 3 | Choose your poison according to your expertise and patient. We generally favour adrenaline, but may add in phenylephrine!! If you have expertise (and good results) with dobutamine, dopamine and/or noradrenaline, good for you! Use what works for you. |
The final section of this document proposes further guidelines that we feel are reasonable. But first, a word of warning!
For years now trauma specialists have hammered home the concept of the "golden hour". Trauma victims, we are informed must be attended to early on and resuscitated adequately as soon as possible, to prevent extreme morbidity and mortality. There is impeccable evidence from many trauma studies that this is the case. Unfortunately, we have neglected to apply this concept in patients with sepsis, who are often more compromised that a trauma patient with a similar blood pressure, and equally impaired vital organ perfusion.
Consider now the patient with septic shock. How often have you seen the following scenario: the patient is found to be in shock. Depending on the level of care prior to this finding, he/she may have been wallowing in septic shock for minutes, hours, or even longer. The attending physician administers fluids (perhaps in sufficiently vast amounts) and after a further delay (often let us say, half an hour to an hour, or even more) this good doctor decides that - eureka - inotropes are required...
Time is taken in preparing the inotrope infusion. Dosages may have to be calculated, drugs drawn up. Then what? Often the doctor, if he is junior and inexperienced, will start an initial, homeopathic infusion of say 0.01 micrograms/kilogram/minute of adrenaline (or say 5 micrograms per kilogram per minute of dobutamine). This will frequently fail, even if the syringe driver pump that the doctor is using is reliable at low infusion rates (many are not)! So, after perhaps five, ten or even fifteen minutes (can it be that he might even delay half an hour or an hour?) the good doctor will tentatively increase the infusion rate a fraction, and carry on.
During all this time, the patient is progressively building up a tissue oxygen deficit, and, basically dying! Can we not apply the trauma concept of a "golden hour" to all patients with circulatory failure? Surely to do anything less is a crime of pure neglect!
Our suggested approach to the patient who may require inotropes:
A. Some Fairly Good References:
| Date of First Publication: 1999 | Date of Last Update: 2006/10/24 | Web page author: Click here |