|
The traditional approach to understanding acid-base is based on use of the Henderson-Hasselbalch equation. This approach is only a partial solution to the problems of acid-base, and therefore breaks down in certain circumstances. The following paper explores some of these circumstances, and how the physico-chemical (Stewart) approach helps us to explain clinical anomalies that are not resolved using a traditional attack. This document is entirely based on an excellent presentation supplied by Jon Waters . |

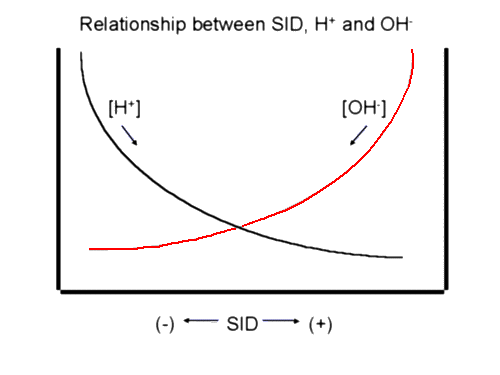
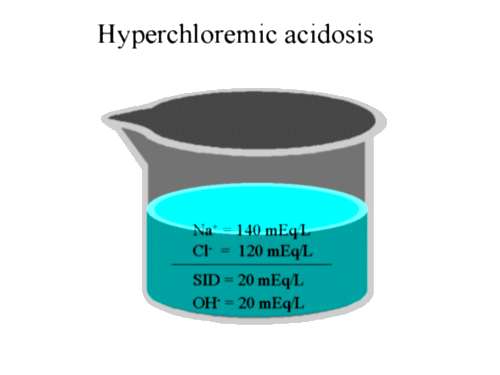
We are now ready to approach acid-base using a physico-chemical model. A very simplistic way of approaching acid-base problems is to think of H + and OH- as charge buffers. Any change in the charge composition of a solution will result in a change in H + or OH- to maintain electroneutrality. For instance, an increase in the negatively charged chloride will result in an increase in H + to maintain electroneutrality. This increased [H +] we call 'acidosis'. Because of the inverse relationship between H + and OH-, it is sometimes easier to assess pH changes through changes in the basic OH-. Increased OH- leads to alkalosis, decreased OH- results in acidosis. For example, the problem of hyperchloremia can be looked at in another way using OH-. The increased Cl- will decrease the SID. The SID is normally positively charged so that the decreased SID would result in fewer OH-. Fewer of the basic OH- results in acidosis. The following picture is a simplified rendering of normal acid-base status in plasma. We will explore changes in acid-base balance using this illustration:
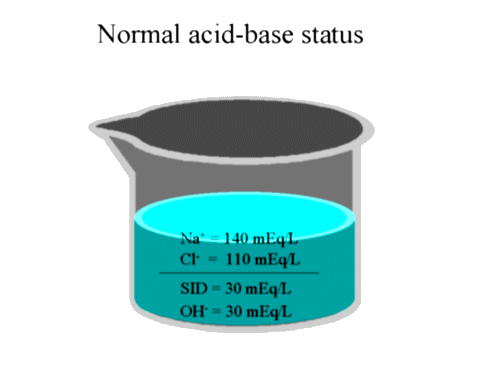 Note that the above picture is used to provide a conceptual framework
for discussion, and is not intended to replace the more detailed analysis
of acid base that we
previously attempted.
Note that the above picture is used to provide a conceptual framework
for discussion, and is not intended to replace the more detailed analysis
of acid base that we
previously attempted.
From the above general approach more specific metabolic problems can be addressed. Metabolic problems arise from abnormalities in either
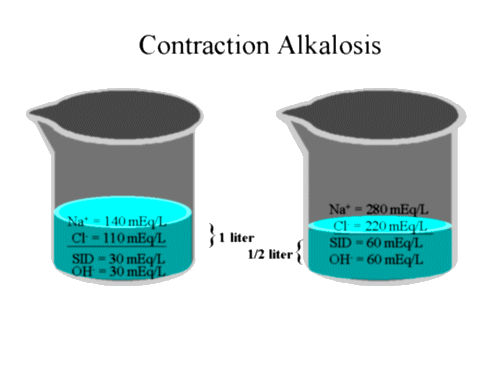
The physico-chemical approach explains the phenomena differently. Changing the amount of free water concentrates or dilutes the electrolytes. By changing the relative concentration of the electrolytes, a dilutional acidosis or contraction alkalosis can result. This change results from change in SID. Development of a dilutional acidosis is best illustrated by an example. If a liter of water contains 140 mEq/L of sodium and 110 mEq/L of chloride than the SID of that solution is 30 mEq. If we were to add another liter of water without adding any more electrolytes, the solution would contain 70 mEq/L of sodium and 55 mEq/L of chloride. Now, the SID is 15 mEq. Because we have decreased the positive charge contribution of the SID from 30 to 15 mEq, a fall in OH- would occur and a "dilutional" acidosis would be seen. This explanation is easily understood by considering the following diagram:
|
Let's further explore the result of adding normal saline to plasma, in the following diagrams: |
|
|
|
Contrast this with the consequences of adding Ringer's lactate: |
|
|
| In the above we assume that (as normally occurs) the lactate is fully metabolised in the liver. This may not apply in severely ill patients, especially those with substantial liver disease. |
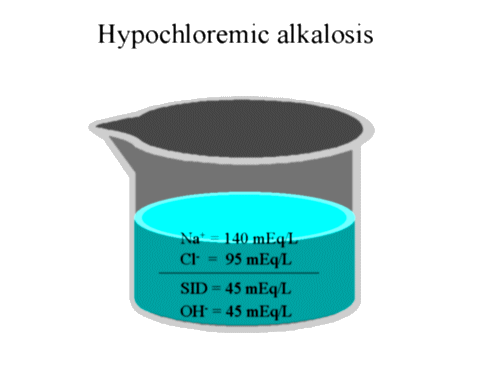
Using the beaker model, treatment can be explained
mechanistically. We would now add one liter of 0.45% NaCl solution containing 77
mEq of Na+ and 77 mEq of Cl-. The final electrolyte concentration would contain
145 mEq of Na+ and 125 mEq of Cl- and an SID of 20 mEq. By the use of this fluid
we have changed the SID from 60 to 20 mEq resulting in a decrease in the OH- and
in fact an over-correction of the alkalosis!
 Administration of normal saline constitutes effective treatment.
This treatment can be illustrated in the same fashion as free
water changes. If we have a one liter beaker of water with 140 mEq/L of Na+ and a
"hypochloremic" 95 mEq/L of Cl- then the SID is 45 mEq. If one liter of normal
saline is added, the beaker would then contain 147 mEq/L of Na+ and 125 mEq/L of
Cl- with the SID being 22 mEq/L. By shifting the SID, we have shifted the pH in the
normal direction. If volume expansion is problematic then potassium, calcium or
magnesium chloride can be administered. The tight regulation and small
concentration of these cations makes them beneficial under these circumstances.
Similarly to
NaHCO3, Cl- could also be administered essentially by itself in the form of
hydrochloric acid (HCl), although most clinicians would shy away from this approach!
Administration of normal saline constitutes effective treatment.
This treatment can be illustrated in the same fashion as free
water changes. If we have a one liter beaker of water with 140 mEq/L of Na+ and a
"hypochloremic" 95 mEq/L of Cl- then the SID is 45 mEq. If one liter of normal
saline is added, the beaker would then contain 147 mEq/L of Na+ and 125 mEq/L of
Cl- with the SID being 22 mEq/L. By shifting the SID, we have shifted the pH in the
normal direction. If volume expansion is problematic then potassium, calcium or
magnesium chloride can be administered. The tight regulation and small
concentration of these cations makes them beneficial under these circumstances.
Similarly to
NaHCO3, Cl- could also be administered essentially by itself in the form of
hydrochloric acid (HCl), although most clinicians would shy away from this approach!
SID can also be affected by the presence of organic acids such as lactate or ketoacids. Again, because these negatively charged molecules lower the SID, they result in an acidosis. Treatment is usually focused on stopping the production of acid. Resolution of the abnormal H + can also be achieved by increasing the SID using NaHCO3.
A Point to Ponder |
| Above, we considered the effect on SID of adding or removing free water to/from plasma. Use your knowledge of the Stewart approach to predict what effect these changes in free water will have on pH by virtue of changes in albumin concentration! |
| Many thanks to Amit Rajguru , MD for his incisive comments on the above page - he pointed out several errors in calculation (which do not however affect the conceptual framework on which this page is based) and also an area where further explanation was required. |
| Date of First Publication: 27/4/2000 | Date of Last Update: 2006/10/24 | Web page author: Click here |