1. Hypothalamus:
thyrotropin releasing hormone
(TRH, thyroliberin, protirelin) <----------------
| \
| |
v |
2. Anterior Pituitary basophilic thyrotropes: <----- NEGATIVE
thyrotropin \ FEEDBACK
('thyroid stimulating hormone', TSH) |
| |
| |
v |
3. Thyroid: T3 -------------------------------------/
(and the "prohormone" T4)
As is often the case in endocrinology, the devil is in the details!
The basic basics
The tripeptide TRH is secreted from the hypothalamus and passes via the portal system to the anterior pituitary, where TSH synthesis and release are stimulated. Thyroid hormones here diminish TSH production through a negative feedback mechanism. The glycoprotein TSH then stimulates receptors on the thyroid gland that promote both:
- Release of thyroid hormones (which then feed back to the pituitary);
- Thyroid gland proliferation.
We now understand the mechanisms of action of the various hormones involved:
- TRH binds to specific receptors and mediates TSH release via G-protein activation;
- TSH binds to its receptors and stimulates cyclic AMP production via a G-protein mechanism.
- The active form of thyroid hormone is T3, which is mainly produced in peripheral tissues from T4 (by deiodination). T3 binds to specific nuclear receptors to mediate most of its effects.
Structurally, the tripeptide called TRH is (pyro)Glu-His-Pro-NH2. Once released from the hypothalamus and transported by the pituitary portal system to the anterior pituitary, the peptide binds to a specific TRH receptor, and TSH is then released. When it binds its receptor, a G-protein is activated - subsequent effects are mediated through activation of phospholipase C. (These effects are antagonised by stimulation of dopaminergic D2 receptors).
Binding of TSH to its receptors increases:
- production of TSH;
- glycosylation of TSH;
- secretion TSH;
Glycosylation is important, because the glycosylated form of TSH is more active, and has a longer half-life in the circulation.
As an aside, TRH is a potent stimulator of prolactin release from the pituitary. TRH production is raised in hypothyroidism, so hyperprolactinaemia is common in hypothyroid women, resulting in oligomenorrhoea or amenorrhoea.
Thyroid hormones appear to inhibit release of TRH, although details are unclear. TRH is inactivated by a specific ectopeptidase called "pyroglutamyl aminopeptidase II" found on the surface of pituitary cells.
TSH, a glucoprotein of molecular weight ~ 28 000, is produced in the basophilic 'thyrotrope' cells of the anterior pituitary. These cells are directly inhibited by circulating T3, and also regulated by T4 levels owing to internal conversion of T4 to T3. There is slow, steady release of TSH through day and night (compare this with the marked circadian variation in e.g. ACTH release).
Several other hormones (for example, somatostatin and cortisol) have also been reported to inhibit pituitary TSH production, although the physiological significance of this is unclear.
TSH has several actions on the thyroid gland. These include:
- Colloid uptake, proteolysis and T4 release occurs within minutes!
- Iodide trapping, peroxidase activity, and glucose metabolism (for NADPH production) rapidly rise;
- The follicular cells become larger (and more columnar), and proliferate over days;
Binding of TSH to receptors is specific due to the structure of its beta subunit (the alpha subunit is shared with other hormones such as FSH and LH). TSH activates a G-protein mechanism, and results in raised intracellular levels of cAMP in thyroid follicular cells, as well as increased phospholipase C activity.
It is best to regard T4 as a prohormone , a precursor of T3. T4 is a highly lipophilic hormone, and also binds serum proteins avidly, much more so than its active form, T3. Synthesis is fairly complex, and there are several points along the way where production may be interfered with.
T4 synthesis starts when the thyroid gland actively traps substantial amounts of the micronutrient iodine. There is a specific ATP-requiring pump that can maintain a concentration of iodine in the thyroid follicular cell that is 30 to 100(+) times the plasma level. (This apical transporter protein is called pendrin - its deficiency in Pendred's syndrome causes hypothyroidism, as well as deafness due to abnormal chloride transport in the inner ear. Note that apart from pendrin, thyroid cells also contain a second iodide transporter called "NIS", the sodium iodide symporter ).
A normal diet contains about 400-500 µg of iodine, of which the gland takes up about 80(+)µg. Once dietary intake of iodide is consistently under 100 µg per day for a period of about three months, hypothyroidism will progressively become apparent. There are vast tracts of land across the planet where iodine deficiency is endemic, so in developing countries hypothyroidism due to iodine deficiency is still a major cause of mental retardation, sometimes worsened by selenium deficiency and goitrogens in foodstuffs such as cassava. Iodine deficiency is said to be a public health problem for about 30% of the world population, and is the most common preventable cause of mental retardation . There are, for example, villages in Zaire where everyone is hypothyroid! In developed countries, we have iodine supplementation of salt.
The rationale behind administering iodine to persons exposed to radioactive iodine (as occurred in the Chernobyl disaster) is not only that orally administered iodine competes for uptake with the radioactive iodine, but also transiently suppresses thyroid activity, the so-called "Wolff-Chaikoff" effect. This effect may be mediated by iodine-induced suppression of proteolysis of colloid.
Iodine intake has other effects - in persons chronically deficient in iodine, sudden provision of large amounts may result in transient hyperthyroidism (the "Jod Basedow" effect). Iodine intake does not necessarily have to be 'dietary' for example, the drug amiodarone has a high iodine content, and can cause hyper or hypo-thyroidism through a variety of mechanisms including the Wolff-Chaikoff and Jod-Basedow effects.
T4 synthesis is interesting - a large tyrosine-rich protein called thyroglobulin (a dimer, MW 660 000) is exposed to iodi n e, which binds to the tyrosine residues. Thyroglobulin accumulates within the colloid of a thyroid follicle. Iodine is produced at the interface between the colloid and its lining of follicular cells by oxidation of iodide, a reaction catalysed by thyroid peroxidase . (The peroxide generated by the peroxidase oxidises the iodide). Iodine reacts with tyrosine residues first to produce monoiodotyrosine (MIT), and then dioodotyrosine (DIT).
.The peroxidase then links two diiodotyrosines to form the two-ringed structure, T4 (monoiodotyrosine and diiodotyrosine can also be linked to form small amounts of either T3, or rT3). At this stage, the T4 is still bound to thyroglobulin, but the follicle cells then actively endocytose thyroglobulin, and lysosomal proteases release T4 (this proteolysis being inhibited by iodine in excess)! Predominantly T4 is released from the thyroid gland (about 80 µg per day, compared with ~4 µg/day of T3). Minuscule amounts of mono- and di-iodotyrosine are released into the circulation - most of the MIT and DIT are deiodinated within the thyroid cells to release iodine (for future oxidation)!
Several drugs inhibit thyroid peroxidase, notably propylthiouracil (PTU), methimazole and carbimazole. PTU also has peripheral actions (inhibits 5' deiodinase) which methimazole and carbimazole do not have . Congenital hypothyroidism may be related to mutations in the thyroid peroxidase, thyroglobulin, and iodide pump (NIS and pendrin) genes. In addition, abnormalities have been reported in TSH receptor genes (autosomal recessive), and TSH itself.
In the healthy individual, 5' deiodinases (five prime deiodinases) in peripheral tissues (mainly the liver) convert about one third of the T4 produced in the thyroid gland to T3, lopping off an iodine atom from the outer ring of T4. (In various disease states, another enzyme called 5 deiodinase may divert large amounts of hormone into production of the inactive rT3, with less production of T3. rT3 is created when an iodine atom is removed from the inner ring of T4).
Details of the control of 5' deiodinase are incomplete, but the activity of this group of enzymes is influenced by:
- propylthiouracil;
- certain beta blockers such as propranolol (different beta blockers have different effects, for example propranolol and alprenolol appear to inhibit 5' deiodinase; atenolol and metoprolol may also inhibit 5 deiodinase activity) ;
- corticosteroids;
- feeding status (starvation inhibits; overfeeding increases activity);
- amiodarone (inhibits hepatic 5' deiodinase);
Note that 5' deiodinase enzymes from the pituitary and other tissues have different properties - the pituitary enzyme has high affinity for T4, rapidly switching off TSH production as T4 levels rise. Conversely, the low affinity, high capacity isozymes in the liver and kidney regulate body T3 levels. Deiodinase isoenzymes are unusual in that they depend on selenium for their activity so selenium deficiency may result in lowered formation of T3 There appear to be three human deiodinase enzymes (deiodinases Type I, II and III). Type I and II have 5' deiodinase activity, removing iodine atoms from the outer ring of thyroid hormones. Type III is a 5 deiodinase (acting on the inner ring) - type I may also have some 5 deiodinase activity. Alternative names for the three deiodinases are D1, D2 and D3.
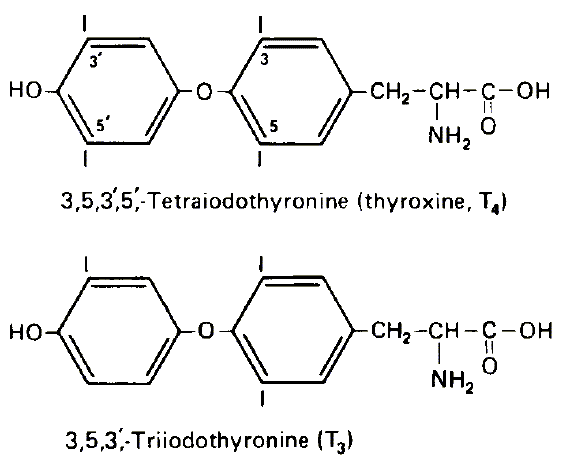
Here are pictures of the T3 and T4 molecules
Thyroid hormone transport in plasma
T4 (and to a lesser extent, T3) bind several serum proteins, mainly:
- thyroid binding globulin (TBG; present in relatively low concentrations, but with high affinity for T4, binds about 2/3 of T4 and T3. Structure is related to that of serum proteinase inhibitors, although TBG has no serpin activity);
- transthyretin (formerly 'thyroid-binding prealbumin', mutants of which are now well-known to cause amyloid neuropathies and cardiomyopathy; also carries retinol-binding protein and thus vitamin A. It is manufactured mainly in the liver);
- albumin. Some albumin mutants may have high total thyroid hormone levels (e.g. a total T4 of twice normal, or more) owing to greatly enhanced affinity for thyroid hormone
Many years ago, physicians did not realise that it's only the free thyroid hormone that is the active form, and so they made errors in diagnosing hyper- and hypo-thyroid states based on total thyroid hormone levels in serum. We now have fairly reliable assays for free hormone levels, so interference caused by different amounts of transport proteins is not usually an issue. Only about 0.02% of T4 (and 0.2% of T3) is free in plasma.
TBG levels are raised in pregnancy (and with oestrogen therapy); genetic and other causes have also been identified (for example, acute hepatitis); levels are lowered with anabolic steroid administration, and high-dose corticosteroids.
Normally only about a third to a quarter of TBG binding sites are occupied by T4 or T3. Thyroid hormone is displaced from its TBG binding sites by several drugs, including phenytoin and aspirin.
The half-life of T4 is about one week, that of T3 is a lot shorter (about 24+ hours) reflecting its lower affinity for transport proteins;
Cellular effects of T3
T3 affects practically every cell in the body, and therefore is a powerful orchestrator of metabolism in the whole organism. The hormone has a potent overall effect on metabolism, although the mechanism of this effect is far from clear. Recent evidence [Endocrinology 2001 Aug;142(8):3414-20] suggests that T3 affects a mitochondrial protein called uncoupling protein-3, increasing metabolic rate by decreasing the efficiency of metabolism! Changes in metabolism may be marked. With profound hypothyroidism, basal metabolic rate as measured by VO2 may drop from 250ml O2/min to 150ml or less; in hyperthyroidism this may increase to 400ml/min. After being actively transported into the cell, T3 binds to nuclear receptors, and alters gene transcription.
Different receptors for T3 occur in different tissues - these all lurk perpetually in the nucleus, waiting for T3 to come along and bind. (T4 can bind these receptors, but only has about one tenth of the affinity). There are two distinct thyroid hormone receptor genes TR alpha, and TR beta. Alternative splicing results in four products (TR alpha 1 and 2, and TR beta 1 and 2; the alpha 2 form is inactive). Mutations in the ligand-binding pocket of the receptor account for most cases of the rare syndromes of resistance to the actions of thyroid hormones.
The nuclear effects of T3 are now fairly well characterised. The combination of receptor and T3 binds to a thyroxine response element (TRE) on DNA, and gene transcription is then altered (decreased or increased). Genes with TREs include:
- growth hormone
- osteocalcin
- myosin alpha chains
- malic enzyme
- TSH
- T3 receptor gene (!)
- .. and many more
T3 also has extranuclear ("non-genomic") effects. Extranuclear T3 receptors occur in:
- Mitochondria - increased activity of mitochondrial adenine nucleotide translocase, apparently unrelated to gene transcription effects;
- ribosomes
- the plasmalemma
Tissue effects of T3 include:
- Increased expression of Na/K ATPase;
- increased sarcolemmal calcium uptake
- increased beta adrenergic receptor levels in myocardium;
- increased hepatic production of sex steroid binding globulin;
- Bone: (cartilage ossification, maturation of epiphyses, chondrocyte maturation; - thyroid hormone has a direct effect on bone, and indirect effects through growth hormone release and IGF-1 action);
- neuronal: cortical growth, axonal and dendritic growth, myelination; T3 is vital for normal brain development, and fetal TSH secretion starts by about 11-12 weeks. The auditory and visual sensory systems appear particularly dependent on T3
- renal: increased renal plasma flow and GFR, Tm in tubules;
There are several interactions between T3 and other hormones, for example:
- Due to increased expression of beta receptors, catecholamine effects are enhanced (notably on the heart);
- corticosteroids and somatostatin inhibit TSH release;
As mentioned above, many factors inhibit 5' deiodinase activity. In the presence of such factors, the amount of rT3 produced will then increase, relative to T3 production. In many disease states, one encounters such a switch. The benefits of this switch (if any) are far from clear, but where attempts have been made to administer thyroid hormone, the results have been harmful. The fancy name coined for this 'syndrome' of diminishished T3 levels is the "euthyroid sick syndrome". With prolonged severe illness, there may even be decreases in T4 levels, and ultimately in TSH levels.
In summary, the rT3/T3 ratio may increase with:
- pregnancy;
- oestrogen therapy;
- fasting;
- liver disease;
- renal disease;
- drugs (PTU, some beta blockers, amiodarone);
- stress, and any cause of catabolism;
Today, the best overall test of thyroid function is to simply determine TSH levels. It is generally a waste of resources to request "full thyroid function tests" including TSH, free T4 and free T3 levels. Current assays of TSH are reliable at both ends of the spectrum - high TSH levels are a sensitive indicator of thyroid hypofunction, and abnormally low levels indicate hyperthyroidism. TSH levels are often raised or low long before clinical correlates of hypo- or hyperthyroidism are noted.
Currently available immunometric assays are sensitive down to 0.1 to 0.2 mIU/ml, or, in the case of "third generation" immunochemiluminometric assays, 0.01 mIU/ml. Even more sensitive assays are in the pipline. Even with "third generation" immunometric TSH assays, it is still vital to correlate findings with clinical evaluation of the patient.
In most laboratories, a normal TSH is in the approximate range of 0.3 to 5 mIU/L, but check with your local lab, and correlate with the clinical picture . In patients on thyroid hormone replacement, TSH levels under 0.2 are associated with osteoporosis and an increased risk of atrial fibrillation.
Tests other than TSH - total T4, free T3 and T4
As mentioned, determining a TSH level alone is often a sufficient indication of thyroid function. Other tests may sometimes need to be performed. Total T4 levels are often determined, but their utility is small. (Sensitive, reliable immunometric assays exist). Of more utility may be determination of free T4 or T3 levels. Free T4 levels may be measured directly by equilibrium dialysis which is expensive and tedious; indirect methods are usually used. Indirect determination of free T4 usually involves assay of total T4, and then determining the amount of T4-binding activity in the sample - free hormone levels can then be estimated. Determination of free T3 levels is similar.
Normal free T4 levels vary from laboratory to laboratory, but are generally in the range of about 7 to 20 ng/L. In the rare circumstances where free T3 needs to be measured, normal values are generally in the range of about 2 to 6 ng/L.
TRH stimulation test
In this infrequently used test, baseline TSH levels are measured. Synthetic TRH is then administered, and a repeat TSH level is taken. (The usual dosage of TRH is 500 µg). This test is mainly of use where hypothalamic disease is supected as a cause of hypothyroidism.
There are several patterns of response - clearly, with pituitary disease as the cause of hypothyroidism, there will be a minimal or absent TSH response to TRH administration. With hypothalamic disease, a TSH response is usually seen, but it is slowed and prolonged. (In primary hypothyroidism, that is, due to thyroid disease, there is an exaggerated TSH response - one can view the pituitary as being 'primed' to produce large amounts of TSH).
The normal response to TRH administration is a brisk rise in TSH levels (by 5-25 µIU/ml within about half an hour), with a return to baseline in about two hours.
Previously, the TRH test was used for evaluation of classical hyperthyroidism, but the only current indications for the test in hyperthyroid patients would appear to be in the rare case with high TSH levels - the TRH test will distinguish between TSH-secreting pituitary tumours, and the pituitary variant of the syndrome of resistance to thyroid hormone ('PRTH', pituitary resistance to thyroid hormone). The latter is quite fascinating - patients present with hyperthyroidism because the pituitary fails to sense the high levels of thyroid hormone. The disorder is usually inherited as an autosomal dominant. Contrast this with people who have generalised resistance to thyroid hormone ('GRTH') - they have high hormone levels, but because all tissues are more-or-less equally resistant, they are clinically euthyroid! The usual abnormality in thyroid hormone resistance is in the beta isoform of the thyroid hormone receptor.
Less frequently used tests
Thyroglobulin levels are occasionally used to determine whether residual thyroid tissue is present in patients who have had thyroid ablation (usually surgery for thyroid malignancy).
Radioactive iodine uptake is an expensive and inconvenient (but sometimes useful) test that may be useful in the differential diagnosis of hyperthyroidism. In patients with thyroiditis, thyroid inflammation may be associated with transient hyperthyroidism that usually settles with time (for example, in "subacute lymphocytic thyroiditis" otherwise known as "silent thyroiditis", which occurs postpartum in up to 9% of women)! In such patients, radioactive iodine uptake will be diminished, in contrast to patients with Graves' disease where iodine uptake will be massively increased.
In the past, there was a proliferation of tests that provided an indirect assessment of free hormone levels. These included the T3 resin uptake, an indirect measure of the level of thyroid binding proteins in serum - as serum thyroid binding capacity increases, so the T3 resin uptake decreases . The reason for this reciprocal relationship is that labelled hormone is added to the patient's serum, and then then the unbound fraction is measured, so with increased binding capacity, the unbound fraction drops. The free thyroxine index [FTI] is simply the T3 resin uptake multiplied by the total T4 level, and is proportional to free hormone levels.
We will not here discuss thyroid gland imaging modalities, and tests for thyroid autoantibodies.
- Notes on a variety of topics are available from thyroid Foundation of Canada
- The Family Practice Notebook has significant information on thyroid function tests.
- David Gardner's page on thyroid function tests.
- The Merck Manual's note on thyroid function.
- A PDF document by Harvey Kaslow on thyroid hormone.
- A fairly good summary from Tiwary clinical laboratory.
-
Dr Bruce Stanley's
 complex (but excellent) lecture from 1999;
[URL: http://www.hmc.psu.edu/core/stanley/4Thyroid.pdf Accessed monday 13 May 2002 13:00].
complex (but excellent) lecture from 1999;
[URL: http://www.hmc.psu.edu/core/stanley/4Thyroid.pdf Accessed monday 13 May 2002 13:00].
- Bryan McIver's PDF on Nonthyroid influences on thyroid function, which discusses amiodarone thyroid toxicity and 'euthyroid sick syndrome' in some detail.
- endocrineweb also has a page on the interpretation of thyroid function tests (relatively simple).
-
Fatourechi V.
Subclinical thyroid disease.
Mayo Clin Proc. 2001 Apr;76(4):413-6
(A contemporary review of subclinical hypo- and hyper-thyroidism)