Dobutamine - Back to Basics
Dobutamine is a synthetic catecholamine that has been in clinical use for over twenty years. Surprisingly, most people who use it are ignorant of its basic pharmacology. Here is a picture of the dobutamine molecule:
As can be seen from the above picture, dobutamine has an asymmetric carbon atom, and can therefore exist as two enantiomers, (-)dobutamine and (+)dobutamine. Clinically used solutions are racemic - a mixture of the two.
Dobutamine pharmacodynamics
Traditionally, dobutamine has been regarded as a fairly selective adrenergic beta-1 agonist. This is far from the truth. Dobutamine does indeed have excellent beta-1 agonist activity, and a small beta-2 effect, but this is less than half of the story!
Dobutamine appears not to have activity at or affinity for dopaminergic receptors, but has complex effects at alpha-adrenergic receptors. The glib, conventional assumption has been that because (-)dobutamine has alpha agonist activity, and (+)dobutamine acts as an alpha blocker, the two effects more-or less cancel one another out in the racemic mixture. This is unlikely to be true.
The alpha adrenergic effects of dobutamine racemate were studied by Kenakin in 1980 [J Pharm Exp Ther 1981 216(2) 210-9]. He found that the racemic mixture was a partial alpha agonist, with a receptor affinity twenty-five times that of noradrenaline (pK A 7.7 vs 6.3), but just over half the efficacy of noradrenaline. Dobutamine antagonises the alpha effect of noradrenaline. (Of interest is that in this study, concentrations of dobutamine required to exert an effect on the beta receptors of rabbit aorta were one thousand times as great as those exerting an alpha effect). Unfortunately, Kenakin then rhapsodises at length about the possible 'non-beta-1 selective' nature of dobutamine, which rather dilutes the impact of his study.
A somewhat better study is that of Ruffolo et al [J Pharm Exp Ther 1981 219 447-52]. These authors looked not only at the racemate, but also the individual stereoisomers. They found that the racemate and (-)dobutamine are potent partial alpha agonists (in agreement with Kenaki's study). (+)dobutamine has about seven times as much beta-1 effect as (-)dobutamine, but is nearly devoid of alpha agonist activity. (+)dobutamine nevertheless has affinity for the alpha receptor that is almost identical to that of (-)dobutamine.
In summary, the dobutamine racemate we use clinically has partial alpha agonist effects. In clinical circumstances where there is marked activation of the sympathetic nervous system, or alpha agonists are used, we can expect dobutamine to antagonise these effects!
Even more intriguing are the effects of dobutamine at the beta-2 receptor. MacGregor et al [Chest 1996 109 194-200] demonstrate convincingly the weak agonist effect of dobutamine at beta-2 receptors compared with full agonists like adrenaline and isoprenaline {although they seem a little confused in that they describe dobutamine as having 'primarily beta-2 adrenergic agonist properties'}. A follow-up article is more exciting [Prielipp R. et al, Anesthesiology 1998 89 49-57]. Here they demonstrate convincingly that both in vitro (lymphocytes) and in vivo (cardiac inotropy), dobutamine competitively antagonised the beta-2 agonist effect of adrenaline! In other words, dobutamine is also a partial agonist at the beta -2 receptor, with about ten times less intrinsic activity at the beta-2 receptor than adrenaline.
The last study suggests that we should be very careful if we use dobutamine in combination with other beta agonists such as adrenaline.
Dobutamine pharmacokinetics
As with pharmacodynamics, there is widespread misunderstanding of dobutamine pharmacokinetics. Dobutamine is always given as a continuous infusion, usually in the range of 5 to 20 µg/kg/min, although rates of up to 200 µg/kg/min have been used by some. Onset of effect is rapid, and removal from the circulation equally brisk (half-life in patients in heart failure reported as being about 2.4 minutes) [Leier C & Unverferth DV, Ann Int Med 1983 99 490-6].
Removal is by the liver. Metabolism is mainly by catechol-O-methyl transferase (COMT) to 3-O-methyldobutamine, with subsequent conjugation to glucuronic acid, and excretion in the urine. (Some dobutamine is excreted as dobutamine glucuronide). Both major metabolites are pharmacologically inactive.
Vast amounts of dobutamine have been used in critically ill patients in recent years. Users should all read the paper by Klem et al entitled Variability in dobutamine pharmacokinetics in unstable critically ill surgical patients [Crit Care Med 1994 22(12) 1926-32]. Clearance of dobutamine from the circulation was first-order. An enormous variation in dobutamine levels was noted at similar infusion rates! For example, at 5 µg/kg/min, serum concentrations of the drug ranged from 25 to 350 ng/mL, a fourteen-fold variation. In addition, clearance changed substantially with time in individual patients. We have redrawn their Fig. 3 to highlight this effect:
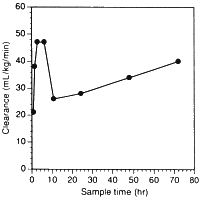
Such variation is not that surprising if one considers the hepatic metabolism of the drug, and that even in normal individuals there is from person to person a five-fold variation in hepatic COMT content. We also can explain clinical studies where titrating dobutamine dose to effect has necessitated infusion rates up to 200 µg/kg/min.
Dobutamine has substantial extra-cardiac effects. This was ably demonstrated in a study by Karzai and colleagues [Brit J Anaesth 1996 76 5-8]. The authors measured oxygen delivery and consumption in patients on cardiac bypass, thus excluding the heart from consideration. A worrying finding was the increase in oxygen consumption seen without any change in oxygen delivery. This implies that dobutamine may stimulate peripheral tissues into increased metabolism, of great concern to those using the drug on critically patients.
It is thought that after more than 72 hours of dobutamine usage, tolerance occurs to the effects of the drug. This may be due to beta receptor down-regulation.
Clinical use of dobutamine
Dobutamine is a valuable agent in clinical medicine. It has a well-established role in the following circumstances:
- Heart failure In acute heart failure, or chronic heart failure that has now decompensated, dobutamine is an excellent agent. It has a positive inotropic effect, with little propensity to increase heart rate. It tends to decrease cardiac afterload, which may also be beneficial.
- Stress testing In the presence of presumed ischaemic heart disease, dobutamine may be used to pharmacologically stress the patient. This is often combined with echocardiography ('Dobutamine stress-echo').
The role of dobutamine in other circumstances is less well-defined. In the 1990's, when 'supra-normalisation' of critically ill patients was in vogue, dobutamine was used to achieve 'supra-normal' cardiac outputs. Enthusiasm for this aggressive therapy appears largely to have waned, although certain individuals still espouse similar practices in, for example, selected patients pre-operatively! There is evidence that use of dobutamine to boost cardiac output may increase mortality [Hayes MA et al. NEJM 1994 330 1717].
Recently we have seen something of a resurgence of interest in dobutamine
administration in critically ill patients for the purposes of 'splanchnic
resuscitation'. We will explore this phenomenon.
'Splanchnic resuscitation'
Many critically ill patients die from multiple organ failure. The
beliefs that motivate the philosophy of 'splanchnic resuscitation'
for such patients appear to be as follows:
- Critically ill patients (especially those in septic shock) may have impaired splanchnic perfusion in the face of apparently normal cardiac output;
- This impaired splanchnic perfusion predisposes to multiple organ dsyfunction;
- Intervention with certain agents will improve splanchnic perfusion and therefore lower the incidence of multiple organ dysfunction, resulting in improved survival.
See for example the editorial by Kumar [Crit Care Med 1997 25(8) 1266-7], and that by Marshall.
We need to look at each of the above assertions in turn. But first let us decide how we are going to assess the effects of vasoactive drugs on the splanchnic circulation.
Defining the splanchnic effects of vasoactive agents
As Kvietys and Granger [Am J Physiol 1982 243 G1-9] pointed out in a fine editorial review, assessment of the effect of vasoactive agents on splanchnic oxygen uptake is difficult. For example, in various preparations, adrenaline has been shown to vasoconstrict and either decrease, increase or have no effect on intestinal oxygen uptake! Part of the reason for this confusion may be that in some circumstances splanchnic metabolism may depend markedly on flow (This is not the normal case, apart perhaps from hepatic metabolism. In several studies, wide variations in intestinal, stomach and pancreatic flow have resulted in tiny changes in oxygen consumption). Bowel distension or baseline hypoperfusion of the gut will result in aberrant results!
Kvietys and Granger recommend the following approach to minimise the confusion that then prevailed in the various studies:
- Establish the relation between blood flow and oxygen uptake in the absence of vasoactive drugs;
- Infuse the drug under both free-flow and constant-flow conditions;
- Estimate capillary exchange capacity (to assess effects on capillary density);
- Assess the effects of the drug on oxygen uptake;
- Consistently use the same dose of drug;
Clinical assessment of splanchnic perfusion
A variety of techniques have been used to assess splanchnic perfusion in critically ill patients. Clearly, the gold-standard - implantation of electromagnetic flow-meters around the vessels in question - is out, so other methods must be sought. These include:
- Indocyanine green (ICG) clearance. This is thought to be a good measure of hepatic perfusion;
- Measurement of gastric intramucosal pH (pHi) or pCO2 gap;
- Laser doppler flowmetry to assess mucosal perfusion;
Unfortunately, all of the above are far from perfect. The meaning and value of pHi have been called into question repeatedly. Contrast editorials by Fink [Chest 1998 114 667-70] 'Tissue capnometry as a monitoring strategy for critically ill patients - just about ready for prime time ' with a review by Brinkert [Int J. Intensive Care spring 1998 16-21] 'Is it time to abandon the pHi concept?'. ICG clearance has its limitations, and laser doppler flowmetry generally assess only a tiny portion of the mucosa - in addition, changes in (for example) the stomach may not mirror what is happening more distally, especially in the colon, a vast reservoir of bacteria. All clinical studies should be seen in the light of the limited methods we have for assessing splanchnic perfusion.
An interesting idea is to look at splanchnic function rather than perfusion. A gross clinical measure is simply whether the intestine is tolerating feeds. (More experimental measures include exotic tests like the use of superconducting quantum interference devices {SQUIDs !} to measure the magnetic field generated by electrical activity in the bowel, a sensitive index of bowel ischaemia).
Animal studies
A variety of animal models have been used to assess the effects of inotropic agents such as dobutamine on the bowel. Results in 'septic' animals have often differed from those acquired in the non-septic state.
'Non-septic' animals
- Priebe et al [Acta Anaesthesiol Scand 1995 39 1088-96] found little effect of dobutamine on small intestinal and total hepatic blood flow in twelve pigs, despite increased cardiac output. With dobutamine, dopamine and noradrenaline no change was seen in hepatic and small-intestinal oxygen uptake.
- The complex effects on the intestine are illustrated by another study [Giraud G & MacCannell KL, J Pharm Exp Ther 1984 230 214-220] where dopamine and adrenaline infusions, titrated to increase intestinal blood flow by 25% resulted a further increase in flow once they were stopped! This hyperaemic response was attributed to shunting of blood from the gut mucosa to the muscle during the infusion, with a compensatory increase in flow with cessation of the infusion. Such shunting seems to be a property of all alpha adrenergic agonists.
- In rats Lee found no benefit of either dopamine or dobutamine on PEEP-induced mesenteric blood flow depression.
Septic animals
- In adequately fluid-resuscitated dogs De Backer found that increases in mesenteric oxygen delivery paralleled increases in systemic oxygen delivery with dobutamine infusion, and VO2 was unchanged. pHi failed to drop in the dobutamine group, in contrast to controls.
- In (tortured) pigs Neviere showed that with (we think) inadequate resuscitation, dopamine may improve gut blood flow.
- In septic pigs Tighe showed no significant benefit of dobutamine, dopexamine or colloid administration on splanchnic DO2 or VO2, despite a 25% increase in cardiac output. Of concern was that pigs who received dobutamine had extensive damage to their liver ultrastructure, something not seen with dopexamine. A problem with this study is that cardiac output was augmented before sepsis was induced!
- In an early study of Breslow septic pigs showed marked vasodilatation, and none of the vasopressors tested (noradrenaline, dopamine and phenylephrine) substantially altered organ flow despite elevations in arterial blood pressure. Of note: "blood flow to the splanchnic organs was not decreased in our study"! (despite marked hypotension).
- In a rather good study on septic sheep Bersten used radiolabelled microspheres to determine regional perfusion. Prior to induction of sepsis, administration of inotropes redistributed blood flow away from the viscera towards the heart, but this did not occur following induction of sepsis. Sepsis decreased vascular responsiveness to a variety of inotropes.
Clinical studies
The above studies don't help very much. What about studies in the critically ill, notably patients with 'severe sepsis'? There are several issues, including the existence of impaired splanchnic perfusion in such patients, whether this actually does predispose to multiple organ dysfunction, and the effects of dobutamine on impaired splanchnic perfusion. Let's ask the questions:
Do critically ill patients have impaired splanchnic perfusion?
Great disparity exists in the literature between various studies that have tried to quantitate hepatosplanchnic blood flow in critically ill (especially septic) patients. For example:
- Meier_Hellmann in a rather peculiarly designed study showed that in patients with septic shock on noradrenaline, splanchnic blood flow was twice as great (2.2 l/min/m 2 ) as in patients with severe sepsis alone (1.3 l/min/m 2 ).
Other reported values have been 0.59 l/min/m 2 in patients on dopamine infusions (Creteur); 0.87 l/min/m 2 , but with values up to 4.7l/min/m 2 (De Backer); and 0.91 +- 0.21 l/min/m 2 (Reinelt).
There is clearly great variation in hepatosplanchnic blood flow in different patients, suggesting that the population is heterogenous. This should serve as a caution against treating all 'septic' patients in the same fashion.
Does impaired splanchnic perfusion predispose to MOF?
Several studies have documented the association between low pHi and poor outcome in ICU. For example:
- In Kirton's small study, failure of pHi to correct at 24 hours (but not 6 or 72 hours!) correlated with poor outcome.
Other studies attesting to the value of pHi include those of Fiddian-Green [Crit Care Med 1987 15 153-6], Gys [Crit Care Med 1988 16 1222-4], Dogilio [Crit Care Med 1991 19 1037-40], Gutierrez [Crit Care Med 1992 20 451-7], Maynard [JAMA 1993 270 1203-10], Marik [Chest 1993 104 225-9], and Trinder [Anaesth Int Care 1995 23 315-21].
Controversy still exists over whether the low pHi reflects localised hypoperfusion, or is symptomatic of systemic acidosis (with which it is often associated). Others have put forward the PCO2 gap (mucosal-arterial carbon dioxide tension) as a better measure of regional hypoperfusion. Recent evidence [Kellum, Crit Care Med 2000, 28 462-466] shows that this is not the case - for detection of a 50% drop in the portal flow of septic dogs, a 20 mmHg gap had a maximum accuracy of 67%, for example. Lactate release by the gut is similarly poorly predicted.
Does dobutamine improve splanchnic perfusion, and if so, in what doses?
Many small studies claim that dobutamine improves splanchnic perfusion. Others show little or no benefit. Here are some of these studies:
- Ruokonen showed no improvement in ICG-determined splanchnic blood flow after dobutamine infusion in a small number of patients with pancreatitis (10).
- Levy took 20 patients with septic shock on adrenaline, and showed that in a subgroup of 13 with an increased PCO2 gap, dobutamine improved pHi.
- Uusaro could find no evidence of redistribution of blood flow towards the splanchnic circulation with dobutamine, in a small non-randomised study of patients following coronary artery bypass grafting. This study may not be relevant to the critically ill, also the case for another small study of theirs in which unspectacular results achieved with infusion of dobutamine into a small number of patients following cardiac surgery result in untrammelled speculation!
- Meier-Hellmann in a crossover study, apparently well designed and performed apart from its size (8 patients), allege that the combination of dobutamine+noradrenaline maintains superior splanchnic blood flow (but note the values: 2.8 l/min/m 2 versus 1.6 l/min/m 2 , with vast variation)!
- Reinelt found that in 12 patients with septic shock where dobutamine was added after stabilisation with noradrenaline, splanchnic flow increased in proportion to cardiac output, and splanchnic VO2 was unchanged, but hepatic glucose production decreased. Baseline splanchnic blood flow (ICG) was 0.91 +- 0.21 l/min/m 2 . There were no controls.
- De Backer tried to address the question of hepatosplanchnic supply dependency in the critically ill. Baseline HSBF was 0.87 l/min/m 2 , with a vast variation, including one value of 4.7 l/min/m 2 !! A most peculiar study.
- Creteur used ICG to determine hepato-splanchnic blood flow (HSBF) in patients on dopamine and found baseline HSBF to be 0.59 l/min/m 2 . Dobutamine increased both cardiac output and HSBF. The main objective of the study was to verify dobutamine infusion as a predictor of low HSBF, which the authors appear to do by dint of complex sub-analyses!
- Duranteau looked at the effects of adrenaline, noradrenaline, or the combination of noradrenaline and dobutamine in twelve patients with septic shock using laser flowmetry. Unfortunately this is a highly selected group (they failed to respond to 20µg/kg/min of dopamine), no baseline measurements appear to have been taken, nor was dobutamine used alone (presumably the blood pressure on dobutamine alone was insufficient). Adrenaline was somewhat less effective than the combination of noradrenaline and dobutamine in increasing gastric mucosal blood flow.
- Levy found no significant effect of either dobutamine or dopexamine on gastric mucosal CO2 gap.
- Neviere's study alleging improved mucosal perfusion and lowered PCO2 gap is unfortunately confounded by the patients' hypotension.
- Parviainen found that with dobutamine administration, increases in splanchnic flow paralleled increases in cardiac output (in patients following cardiac surgery). pHi failed to improve with dobutamine therapy, and often decreased! (This study may be of limited applicability to the critically ill).
- Gutierrez shows that with time and dobutamine, pHi increases in septic patients, unfortunately without controls.
- Although much-quoted, the retrospective study of Silverman, which allegedly shows the superiority of dobutamine to red-cell transfusion in the management of sepsis appears grossly flawed.
- Leier [Am J Cardiol 1988 62 86E-93] reports that in human heart failure, dobutamine's main vasodilating actions are seen in limb musculature, with little effect on hepatosplanchnic flow.
- A recent, large, apparently well-designed study [Gomersall et al, Crit Care Med 2000 28 607-14] compared titration of dobutamine and colloids against controls in 210 critically ill adults, with pHi as an end-point. The intervention resulted in no benfit.
In summary, there is no clear evidence that dobutamine has a consistent, beneficial effect on splanchnic perfusion in critically ill septic patients. Most studies that try to investigate such effects are small, and many are flawed. In many studies, fixed doses of 5-10 µg/kg/min of dobutamine were given; in others, administration was titrated to effect. In the single large study where 'splanchnic resuscitation' efforts were titrated against pHi, no benefit accrued.
Does dobutamine administration lower the incidence of MOF?
This is the million dollar question. Large well-designed studies are needed - the only study we are aware of that approximates these requirements, that of Gomersall et al, showed no benfit, with similar ICU and hospital mortalities in the two groups.
Conclusion
Dobutamine is an agent with complex actions. From basic studies of its pharmacology, we expect pronounced beta-1 effects, minimal beta-2 agonist activity (with the potential for beta-2 antagonism), and some alpha adrenergic agonist activity (with the potential for profound alpha-adrenergic antagonism in hyper-adrenergic states). Elimination of the drug is highly variable both in and between individuals - dose should probably therefore be titrated to effect, something which has not been done in many studies.
Critically ill patients constitute a heterogeneous population. There is no consistent evidence that overall hepatosplanchnic flow is consistently low in such patients, in fact, this flow may be increased in many critically ill patients with sepsis. Mucosal blood flow need not necessarily correlate with total bowel blood flow, and imbalances in flow may be exacerbated by adrenergic agents with alpha agonist activity.
There is no clear and consistent evidence that dobutamine (or any other agent) has a beneficial effect on hepatosplanchnic blood flow or intestinal mucosal flow, although some studies suggest this. More important is the current lack of evidence that administration of dobutamine has a beneficial effect on outcome. Some studies where dobutamine was titrated to effect in critically ill patients suggest that administration of the drug may be deleterious.
There is every reason not to add 'low-dose dobutamine' to management of the critically ill until convincing benefits are shown in decent-sized studies. It is unclear (given the wide variation in blood dobutamine levels at a given infusion rate) how such administration would be titrated to effect. The use of pHi seems questionable in this regard. Based on our current knowledge, routine administration of a fixed dose of dobutamine to critically ill patients with impaired hepatosplanchnic blood flow would appear extremely ill-advised.
References
We have appended a list of relevant references including brief informal reviews of the articles, as a separate file.
| Date of First Publication: 2000-7-16 | Date of Last Update: 2006/10/24 | Web page author: Click here |