 Sketch of heart muscle cells.
Sketch of heart muscle cells.
The anatomy of the heart muscle cell (cardiac myocyte) fits its physiology almost as well as the intercalated discs at the end of the cells fit one another! The extensive branching and interdigitation of the cells helps to provide mechanical cohesion of the cells, but more than this, it provides a substrate for electrical cohesion. Associated with the intercalated discs is a vast network of "gap junctions" linking the sides of the muscle fibres and providing a low resistance pathway for the rapid conduction of electrical current. Heart muscle functions as a syncitium, despite the fact that it is made up of discrete, individual cells!
 Sketch of heart muscle cells.
Sketch of heart muscle cells.
Description of the myocyte is unnecessarily complicated. This is because
the light microscopists who initially prepared sections of muscle noted
a variety of different "bands" and "lines", without understanding the
underlying structure, best seen with electron microscopy. We now know that
the 'motor' inside the muscle cell is made up of interdigitating fibres
of actin and myosin. This is true for both skeletal muscle and cardiac
muscle, although several differences between the two exist. Actin filaments
are thin, myosin are thick. The thick myosin fibres have many projecting
heads, and it is these tiny heads that swivel backwards and forwards, moving
along the actin fibres like a dying cowboy crawling into the distance along
a railway track, inch by painful inch (or in this case, in minuscule steps of
about 11 to 15 nanometres, exerting a force of just 3-4 picoNewtons).

The sarcomere is the "fundamental unit" of the muscle cell. It has a Z disk at each end, and the thin actin filaments extend from both Z disks in towards the centre of the cell. The thick myosin filaments are found towards the centre of the sarcomere. We can see where all the microscopic complications arose: the Z band corresponds to the Z disk, a light I band is actually made up of actin filaments from two adjacent sarcomeres, cut in two by a Z disk, and the dark M band is made up of thick myosin filaments in the middle of a sarcomere.
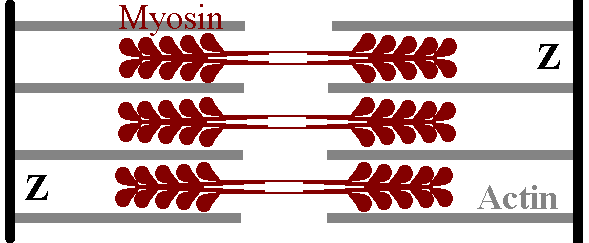 How myosin and actin are
positioned.
How myosin and actin are
positioned.
{ A note on terminology: where do all these crazy initials come from? Well
the Z-band is short for Zwischenscheiben, which translates as something like
"interval disc", the I band is so named because it is isotropic in polarized
light, while the A band is anisotropic. The dark A band is bisected by
the lighter H (Heller) band, which in turn is cut in two by the denser
M (Mittelscheibe) band. Consult a good German dictionary, yes!}.
Differences are numerous, including:
We now know a fair bit about how heart muscle functions. The key components, as mentioned, are actin and myosin, but there is an exquisitely fine tuning system that ensures appropriate control of contraction. Key regulatory element include troponin and tropomyosin, and these in turn are controlled by the concentration of intracellular calcium ion (Ca ++). Ca ++ is released from the sarcoplasmic reticulum of the myocyte, and then re-absorbed to terminate contraction of the myocyte. Sudden release of calcium occurs when the myocyte cell membrane depolarises. There are thus several important questions we have to answer before we even begin to understand how heart muscle functions:
Cardiac myocytes are packed with mitochondria, to provide the energy for the perhaps two billion heartbeats that will occur during a human lifespan. Metabolism is almost exclusively aerobic - heart muscle does not tolerate lack of oxygen well. Energy comes from a variety of sources, but the most important one is fat, providing about 60%, with carbohydrate coming in second at 35%. Ketones normally only provide about 5% of the energy, although these proportions vary with the nutritional state of the organism. For example if large amounts of glucose are ingested, lactate and pyruvate utilisation may increase. Fifty percent of the energy derived from fat comes from circulating free fatty acids. The ferocious utilization of energy by the myocyte is also reflected in the abundant capillaries coursing through the myocardium, and the large amount of myoglobin within heart muscle.
Where does all the energy go? Adenosine triphosphate (ATP) is the energy
"currency" of the cell, and it is this that is hydrolysed when the tiny
head of a myosin molecule swivels, dragging itself and ultimately the
whole myocardium along the actin railroad track! Actin catalyses the
hydrolysis of ATP by the ATPase on the myosin head - from a resting
rate of 4 molecules per hour, up to 20 molecules per second.
|
|
Myocardial contraction is initiated by membrane depolarisation. For this reason, and because abnormalities of depolarisation result in an impressive array of often life-threatening disorders, we must understand it well.
Depolarisation of the normal myocyte occurs as follows:
|
|
Consider this example of uncontrolled positive feedback: more depolarisation results in yet more Na channel opening, which results in more depolarisation, and so on.. From the many physiological systems where this sort of thing occurs, we can extract a general principle - that the process which initiates such massive positive feedback almost always initiates a slower counter- regulatory process. Unsurprisingly, this occurs here - and the counter-regulatory process closes the sodium channels, resulting in Phase 1 of the action potential. Just to make sure, an influx of chloride occurs at about the same time.
The broad Phase 2 plateau which now follows is due to a totally different mechanism. While the above has been going on, Ca ++ channels have been slowly starting to open, and it is this influx of calcium that causes the plateau. Finally these close, and to make sure of things and return the voltage to its previous negative levels, potassium ions are permitted to rush out of the cell. There are at least eight different potassium channels that may play a role in this Phase 3 repolarisation.
At this point consider taking a brief glance at our insubstantial ion channel page!
Skeletal muscle can undergo a tetanic response with repeated stimulation. Presumably, those organisms whose hearts easily went into tetanic spasms died out several hundred million years ago, when cardiac muscle first started appearing on the evolutionary scene. Indeed, there are several mechanisms that specifically prevent the heart from being repeatedly stimulated in this fashion. The most important is the "refractory period" - from phase 0 until about half-way into phase 3, heart muscle cannot be excited again - it is absolutely refractory to stimulation. Nothing is perfect however, and for a short period after this (during the relative refractory period) a critically timed stimulus may result in ventricular fibrillation and death. This is important in a variety of heart diseases, as well as being critical to the understanding of how electrical shock can kill someone.
How does all of the above relate to sarcomere contraction? As we have
mentioned, the intracellular concentration of calcium ion,
commonly abbreviated to [Ca ++]i , is a vital
regulator of interaction between actin and myosin. Most of the calcium ions
that regulate this process come from the sarcoplasmic reticulum.
Depolarisation initiates a rapid release of calcium, the rate of which
has a bell-shaped dependence on membrane potential - at positive
membrane potentials, release diminishes rapidly. Interestingly,
in heart muscle, calcium can be released on repolarisation from positive
membrane potentials! The initial burst of calcium release is inhibited by ryanodine which locks the sarcoplasmic calcium release channel
into a subconducting state. Abnormalities of this ryanodine receptor
are implicated in the pathogenesis of the  malignant hypermetabolic
syndrome.
malignant hypermetabolic
syndrome.
The sarcolemmal Ca ++ ATPase pump is probably unimportant in affecting intracellular calcium levels, and sarcolemmal calcium channels also have a minimal effect on intracellular calcium concentration, at least in the mammalian ventricle. [ Wier WG Ann Rev Physiol 1990 52 467-85pp] The electrogenic Na/Ca exchange mechanism may not be important in transient changes of [Ca ++]i , but probably is an important mediator of Ca ++ extrusion from the cell. The role of so called "calcium-induced calcium release" in the heart is still unclear, at least to this author!
Re-uptake of calcium ion into the sarcoplasmic reticulum is probably mainly mediated through a sarcoplasmic calcium ATPase pump. Binding to intracellular calcium ligands is probably important in regulation of [Ca ++]i transients.
Myosin is a mechanochemical enzyme, converting the energy stored
in the high-energy phosphate bond of ATP to useful work, at the
cost of hydrolysis of the ATP.
As mentioned, the principle controlling mechanism of actin:myosin
interaction is [Ca ++]i . Calcium binds
to the thin filament, and tiny changes in calcium concentration
profoundly alter the interaction between actin and myosin.
No contraction occurs with depletion
of intracellular calcium, there is about a 50% response at concentrations
of 2 micromol/l, and doubling this results in about 90% activation.
{Technically, a Hill coefficient of over 3}. The regulatory molecules
are tropomyosin and troponins C, I and T.
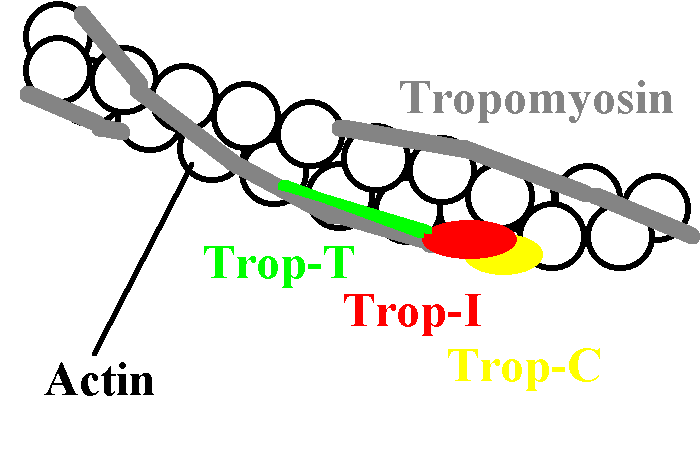 A thin filament
A thin filament
Troponin C has four metal-binding sites, but it is site II that primarily
regulates cardiac contraction in heart muscle.
Troponin C inhibits the interaction of actin and myosin, provided there
is no Ca ++ around. Once calcium concentrations rise, the
inhibition decreases, and the two interact to cause motion.
This inhibition does not only depend on Troponin C. Tropin I must also
be present. Troponin T is the glue that holds the whole system together.
There are several differences between skeletal and cardiac muscle:
myocardial troponin T is shorter than that in skeletal muscle, cardiac
troponin I also differs slightly, and sites I and II are both active
in skeletal troponin C. Troponin binding to actin+tropomyosin is usually
modelled using the "two-site model", although a "three-state model" has
been postulated.
The tropomyosin molecule seems to mediate the long range effect of troponin, as there is only one troponin for every seven actin molecules. Tropomyosin spans seven actins!
As an aside,
severely injured heart muscle releases a variety of substances, including
troponin T, and assays are now available to detect this.
Mechanical influences also play a major regulatory role. The most well-characterised is that which mediates Starling's law of the heart: as preload on the fibre increases, so contraction becomes more forceful, and the amount of blood ejected increases. This makes sense: if more blood enters the ventricle during diastole, stretching the fibres, then more should leave due to the more forceful contraction. Note that at very high fibre lengths, further increases in volume will NOT result in increased force of contraction, but will in fact result in a decrease. This is the infamous "descending limb of the Starling curve" - there still seems to be argument as to whether this is clinically relevant. Experimentally, this descending limb appears to not be related to lack of overlap of actin and myosin in the cardiac sarcomere (as occurs in skeletal muscle) but rather to actual disruption of myocardial fibres! [Ganong, 17ed p 70]. {It is also possible that mechanical factors may have a profound effect on ion channel function or expression, thus even altering predisposition to arrhythmogenesis}.
Catecholamines increase the force of contraction of
the heart without any change in muscle stretch. The beta-1 receptor was
considered pivotal - we now know that other receptors are also important.
The cardiac beta-1 receptors are innervated, and respond to both sympathetic
stimulation and circulating catecholamines by increasing intra-cellular
cAMP levels. Non-innervated beta-2 receptors also exist, but their
contribution to inotropy is uncertain,
and this may be mainly at atrial level. Alpha receptors on the myocardium
may be much more important than previously thought.
{ Alpha-adrenergic
drugs stimulate production of diacylglycerol (DAG) and inositol triphosphate
(ITP), as well as activating the Na +/H + antiporter
which increases sensitivity to calcium ion by raising intracellular pH.
DAG may stimulate phosphokinase C, increasing myofilament sensitivity
to calcium ions, while ITP probably increases calcium release from the
sarcoplasmic reticulum.
There is a forest of other receptors that can influence contractility, including beta-3 receptors, angiotensin II receptors, and even endothelin receptors. The last two both have positive inotropic effects, but these may be more than counterbalanced in the long term by other nasty effects, such as adverse effects on cardiac remodelling, and coronary vasoconstriction. }
Stretched muscle develops a resistive force, similar to an elastic band. Extremely long muscle proteins account for this. Perhaps the most important is titin , a 2.7 million Dalton fibrous protein that links the ends of the myosin molecule to the Z disks on either side, also keeping the myosin centred within the sarcomere. In skeletal muscle, another protein called nebulin regulates the position of thin filaments, but this is absent from cardiac muscle.
There appears to be much information on the web, if you start searching for terms such as "cardiac myocyte" using the popular engines. A lot of this is however people boasting about what they have done (without specifics) or saying what they are about to do. Best stick to the printed literature. You could try:
| Date of First Publication: 2000 | Date of Last Update: 2006/10/24 | Web page author: Click here |