(A short note)
Neonatal jaundice can be a significant problem. There are four main reasons for this:
Even a moderate insult can tip a neonate over the edge into severe unconjugated hyperbilirubinaemia, with poor feeding, decreased production of urine and stool, and a vicious cycle of worsening jaundice. The end result may be kernicterus , a condition where bilirubin causes toxic destruction of nerve cells in the basal ganglia. This may manifest as lethargy, hypotonia, and seizures, followed by (Phase 2) hypertonia, opisthotonos and fever, and then (Phase 3, after about a week) marked hypertonia. The long term sequelae are devastating - delayed motor skills, abnormalities of tone and reflexes, culminating in cerebral palsy (especially athetoid CP), and frequent deafness. With severe haemolysis, kernicterus occurred in 8% of babies with bilirubin in the range 19 to 24mg%, one third of those with bilirubins of 25-29mg%, and in three quarters of those with levels of 30 or more. (Dennery et al, ref 37). Pathologically the name 'kernicterus' comes from the yellow staining seen in the basal ganglia at post-mortem.
We will briefly explore some causes of hyperbilirubinaemia. Note that the following brief notes should NOT be used to dictate therapy (which should be performed under the guidance of an expert neonatologist). They are for educational purposes only.
Neonatal hyperbilirubinaemia is usually unconjugated. This is for the reason already mentioned - hepatic conjugation of bilirubin to form bilirubin mono- and di-glucuronide is minimal at birth, and the enzyme system takes several weeks to become fully functional. Consequently, many normal infants have a degree of "physiological jaundice ", which often peaks on about the third day after birth. About 5 to 10% of neonates may develop bilirubin levels of more than 10mg%. It is said that even at 37 weeks of gestation, infants are four times more likely than a term infant to have a serum bilirubin over 13mg%!
Hyperbilirubinaemia is more common in infants born at altitude! [Am J Dis Child 1984 Feb; 138(2): 157-61]. Some population groups appear predisposed to neonatal jaundice, for a variety of reasons, including a mutation in UDP glucuronsyl transferase (Asians), and a high incidence of glucose 6-phosphate dehydrogenase deficiency (Greeks, ..). Most authorities seem to agree that a bilirubin above 17mg% is definitely not 'physiological', although this is clearly an arbitrary cutoff.
There are numerous reasons why infants might develop severe un conjugated hyperbilirubinaemia. Formerly, before the problem of Rhesus (Rh) incompatibility was recognised and addressed, this was a relatively common cause of major foetal morbidity and mortality. Mothers would become immunised to the Rh antigen when small amounts of foetal (Rh positive) blood leaked into the Rh-ve maternal circulation, and with the next pregnancy, the mother would produce vast amounts of Immunoglobulin G, which crossed the placenta to cause massive haemolysis in the foetus. If the baby survived to be delivered, it would often be severely anaemic, grossly jaundiced, and critically ill, with frequent neurological consequences. Now, with the routine administration of anti-D whenever there has been a suspicion of leakage of Rh+ve foetal cells into the circulation of the Rh-ve mother, Rh incompatibility should be rare. (The anti-D destroys the Rh+ve cells before maternal sensitization takes place).
There remain other pathological causes of unconjugated hyperbilirubinaemia in the infant. Perhaps the commonest is ABO incompatibility - some Group O mothers will produce IgG antibodies to the A or B blood group antigens (commonly A), and these may cause a picture similar to Rh incompatiblity, but often milder. Any other cause of increased breakdown of red blood cells can produce a similar picture. Such causes include:
In addition, some infants may have markedly impaired liver conjugation of bilirubin. This occurs in:
In yet other disorders, the hyperbilirubinaemia is poorly understood, or multifactorial. Conditions include
Maternal milk jaundice is interesting and complex. Neurological sequelae are said to be uncommon as the jaundice is usually moderate. There are two considerations:
Clearly, it is not just sufficient to treat neonatal hyperbilirubinaemia - the cause should be found. Often, a thorough history from the mother and examination of the infant will reveal a likely cause. ABO incompatibility in a previous infant is likely to be followed by jaundice of a similar degree in subsequent ABO incompatible neonates. If jaundice due to ABO incompatibility is anticipated, it is extremely useful to have a sample of cord blood available, so that infant blood group can be determined, and a Coombs' test performed if the infant is A, B or AB. Apart from determining infant levels of conjugated and unconjugated bilirubin, it may be appropriate to do a full blood count (CBC) with a blood smear for red cell morphology, and other tests, depending on ethnicity and clinical suspicion of red cell haemolytic disorders. Note that with ABO incompatibility, a negative Coombs' test does not exclude haemolysis, as this test is infrequently positive, although a positive result may suggest more severe haemolysis. Others even check the serum albumin levels, as they regard low values as a risk factor for more severe consequences of hyperbilirubinaemia. (This makes sense, as each mol of albumin can bind one mol of unconjugated bilirubin. If the albumin level is 30g/dl, then 25mg/dl of bilirubin is the maximum binding capacity).
Clinical assessment of the degree of jaundice is often poorly correlated with serum levels, however it is well recognised that as the severity increases, so yellow pigmentation spreads from the face to the trunk and eventually the extremities. If there is staining of the feet, a serum bilirubin is most advisable. Transcutaneous bilirubinometry has been used to screen for infants with significant hyperbilirubinaemia. End-tidal carbon monoxide levels have been described as a measure of bilirubin production, although this test is not yet widely available.
Finally, most authorities agree that if a neonate is discharged early from hospital, he/she must be checked for jaundice by a competent practitioner on day two or three post-partum.
Generally, premature infants are at greater risk of kernicterus, and the tendency is to start treatment of hyperbilirubinaemia at lower levels of bilirubin. Note that still today, bilirubin levels are commonly expressed in the archaic "milligrams percent" (mg/dl), as paediatricians are familiar with such levels. The conversion factor is seventeen - for example, a level of 20mg% is the same as 340µmol/litre. It has generally been recommended that in healthy term infants, bilirubin levels be kept under 20mg%. It is commonly believed that infants with haemolysis are at greater risk of kernicterus than infants with similar levels of bilirubin, without haemolysis. Other worrying risk factors include:
There are no clear, well-validated recommendations as to when phototherapy should be started. The American Academy of Pediatrics have made recommendations for term otherwise healthy neonates that suggest commencement at levels varying from 15mg% (levels at 25 to 48 hours), to 20mg% (72 hours or more) but these have been criticised as being too relaxed. There seem to be absolutely no well-founded recommendations for neonates who are premature, or those with haemolysis, although lower threshold levels would seem highly advisable. (Some have advised phototherapy at all levels over 10mg% in well prems, or term but ill neonates, and even more aggressive management in ill premature babies, for example at levels of just 5mg%. In suspected ABO incompatibility {maternal Group O}, some authorities perform a serum bilirubin at 6 hours post-delivery, and if this is over ~4.7 mg% (80 µmol/l), phototherapy is started).
There have been reports of kernicterus in term infants with hyperbilirubinaemia of 20mg%, and no other 'risk factors' - such babies were commonly discharged early on, and breast-fed. Such cases have raised concern about the risks of watchful management of hyperbilirubinaemia (See for example the Canadian statement). It is important to note that all recommendations are to a certain extent empiric, in that they have not been validated in prospective controlled trials (nor is this ever likely to happen, for obvious reasons).
Modern phototherapy stems from the fortunate synergy of a nurse who noticed that babies nearer the windows of a nursery in Essex were less jaundiced than those further into the room, and a physician who was smart enough to listen to his nurse! We unfortunately don't know the name of the observant nurse* who has saved so many people from the ravages of severe hyperbilirubinaemia, but the physician was RJ Cremer. Phototherapy caught on rapidly after his initial report (apart from in North America, where delays were substantial).
*Thanks to Vincent Harrison, who in 2006 provided us with the name of the nurse --- Sr J. Ward
Conventional phototherapy works . There is no doubt that blue or green light of sufficient intensity effectively causes photoisomerisation of bilirubin to products such as the water-soluble lumirubin, rapidly excreted in bile (and urine). There has been much argument about refinements to the technique of phototherapy, for example use of high intensity monochromatic laser light to rapidly bleach the skin of bilirubin. (An argon laser, for example, produces light of the appropriate wavelength).
Clearly, the effectiveness of phototherapy depends not only on the wavelength and intensity of the light, but also on duration of therapy and area of skin exposed to light. The greater the bilirubin levels in skin, the more effective phototherapy will be. Practically, important points in phototherapy are:

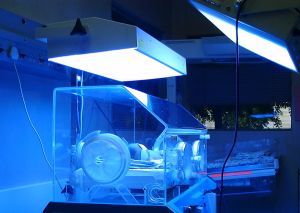
| Fig 1. In the above picture of a neonate in an incubator 'under lights' note that the right hand phototherapy unit is a bit too far from the infant, and that white sheets around the units would increase overall light delivery. |
There is a wide variety of devices available for phototherapy, but no standardisation. Paediatricians seem generally to have gone for the philosophy of "using what seems to work", but it is highly likely that coventional phototherapy uses excessive doses to achieve less than optimal results. A recent paper by Dicken et al [Physiol Meas 2000 Nov;21(4):493-503] reviews the chaos.
Some have characterised therapy as 'low' or 'high' dose based on light intensity. See [Pediatrics 1985 Mar;75(3):519-22]. Levels of up to about 40 (or even 200) µW/cm 2 /nm have been used. The minimum effective energy is perhaps 6 µW/cm 2 /nm, but note that this must pertain in the appropriate frequency range! Intensities of about 12(+) µW/cm 2 /nm are commonly achieved with conventional phototherapy. Some actually measure the light intensity at the skin.
Phototherapy acts on bilirubin to a depth of 2mm into the skin. The mechanisms of removal of bilirubin are interesting. Here is a picture of the bilirubin molecule:
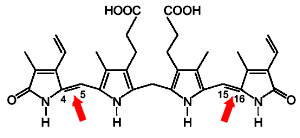
| Fig 2. The bilirubin molecule. |
This 'normal' isomer is sometimes called the 4Z-15Z, or 'ZZ' isomer. The reactions that occur with light include cis-trans isomerisation about the C 4 -C 5 and C 15 -C 16 double bonds arrowed above, photo-oxidation, and occurrence of an intra-molecular cyclisation (to form lumirubin).
The exact light frequency best used is still debated. Some research suggests that lumirubin production is optimal with blue-green light at a frequency of 490 to 510 nm [Agati et al, J Photochem Photobiol B:Biol 17:197-203 (1993)]; others suggest (eg. Myara, [Biol Neonate 71 (2): 75-82, 1997]) that blue light is optimal, with the production of other photoisomers such as 4Z-15E, and the similar 4E-15Z isomer. The latter researchers do not however address whether the photoisomers they favour are efficiently removed by the neonate, whereas lumirubin is . It is thought that the (irreversible) production of lumirubin from ZZ bilirubin is via the 4E15Z isomer. Lumirubin appears to form best when bilirubin is bound to albumin. Practically, blue and green lights appear to have similar effectiveness, despite green light penetrating skin more effectively. The bias in favour of blue (or intense white) light seems to have more to do with avoiding the lurid skin colour seen with green light, than real science!
Note that laboratory assays for "bilirubin" include circulating photo-isomers. As these are thought not to be neurotoxic, bilirubin 'levels' reported on phototherapy may well be 'overread' due to the mix of nontoxic photoisomers with the toxic ZZ isomer!
These appear to be tiny. There may be potential for retinal damage if the eyes are not shielded, and blue light potentially may cause cellular damage (including damage to DNA) [Acta Paediatr 1994 Jan;83(1):7-12]. Some units use 'cut-down' nappies, so male gonads are shielded. (Although nobody knows whether long term damage might result, the risks seem small, and blue light doesn't penetrate very deeply). Dehydration shouldn't be a problem if hyperthermia is avoided. Increased skin blood flow may occur (not usually a problem), and stools may be more loose than normal.
Authorities vary as to the level at which phototherapy may be discontinued. Most give values of about 4mg%, or less. There is less than convincing evidence that a "rebound hyperbilirubinaemia" occurs after discontinuation of phototherapy, but one should still watch the infant very carefully, especially where an underlying haemolytic condition is present (such as glucose 6 phosphate dehydrogenase deficiency).
Exchange transfusion involves simultaneously removing small volumes of patient blood, and transfusing similar volumes of donor blood mixed with plasma. Ultimately, two whole blood volumes are replaced! The procedure is effective, but substantial potential complications in up to 12% include:
There is no exact cutoff level above which exchange transfusion is mandatory, as the risk factors and general state of the infant have to be taken into consideration. Generally it is performed if:
A variety of other options has been tried to limit hyperbilirubinaemia. Such options include:
Although we usually shy away from personal anecdote, we will just this once succumb to temptation. Our first child was (in retrospect) distinctly yellow on day 1. He soon developed unconjugated hyperbilirubinaemia of 25 mg%, with a dramatic response to phototherapy, rapidly instituted by a paediatrician who was a clued-up and competent lady. He escaped exchange transfusion and long-term sequelae. The jaundice was almost certainly due to ABO incompatibility, so we were decidedly jumpy with our next pregnancy, and asked that cord-blood ABO typing and Coombs be performed when our daughter was born. The child was well-grown but 4 weeks prem, and group A like her brother. Eight hours after the birth, she looked yellow, so we persuaded the reluctant paediatrician that a serum bilirubin was a good idea. This came back at 5.6mg%, so we bludgeoned the ever more reluctant man into ordering phototherapy. In the face of phototherapy, over two days the bilirubin rose to 9.9mg%, and he nevertheless discharged her the following day (after a further night of phototherapy, continued by the nurses despite his saying that it was unnecessary). Over the next two days, the bilirubin peaked at 16.1 mg%, and then fortunately settled. To this day, we're not sure that we did the right thing, (Don't you just hate doctors who interfere with the management of their family members?) but are fairly certain that without the phototherapy, our daughter would have duplicated her brother's problems (including a three day stint in ICU). Hence the web page, written by two doctors who are decidedly not paediatricians, but wish to highlight the medical confusion that seems to abound in the management of neonatal unconjugated hyperbilirubinaemia!
Have you wondered:
 available on the web.
available on the web.
| Date of First Publication: 2001/11/25 | Date of Last Update: 2006/10/24 | Web page author: Click here |