How arrhythmias arise
The normal rhythmicity and error-free operation of the heart might be considered a small miracle, given the complexity of the system! Unfortunately when things do go wrong, they frequently go seriously wrong - many of us will die an arrhythmic death. There are many different ways to classify heart rhythm disturbances. In managing these arrhythmias, we believe that one should be practical, and ask the following questions:- Is the arrhythmia fast or slow?
- Is the origin of the rhythm disturbance ventricular or supraventricular?
- Is the patient haemodynamically compromised?
- Does the arrhythmia need management?
- What is the underlying substrate that predisposed to the arrhythmia?
- What triggered the arrhythmia?
- Will the arrhythmia recur?
'Blocks and bradys'
One manifestation of disease affecting the myocardium may be impairment of automaticity or conduction. We are fortunate in that generally if one pacemaker fails, another normally subsidiary pacemaker takes over, usually at a lower rate. Many factors can impair pacemaker automaticity or myocardial impulse conduction - drugs such as beta blockers or sodium channel blockers, electrolyte and pH disturbances, myocardial ischaemia or hypoxia. (One always remembers that under anaesthesia, the first ten causes of bradycardia are all hypoxia!). Anything that enhances parasympathetic tone will likewise inhibit the rate of impulse formation and conduction. Unfortunately, it's the much more sexy tachyarrhythmias that have excited most interest - but don't forget that hundreds of thousands of pacemakers are inserted every year for bradyarrhythmias!
Tachyarrhythmias
Despite the bewildering variety of tachyarrhythmias, there are just three basic mechanisms, and of these, one predominates. They are:
- Reentrancy
- Increased automaticity
- Triggered activity
Of these, re-entrancy seems to be coming more and more to the fore! The basic concept is simple - an advancing impulse reaches a point (let's call it X) where the wavefront has the possibility of moving along two different pathways - as if it had come to a fork in the road. Unfortunately, the impulse is blocked as it moves down the one limb (A), and passes unhindered down the other limb (B). The key point is that the two limbs then join up again, and at this point the impulse moves retrogradely up limb A. When the impulse reaches point X again, limb B is again able to conduct the impulse downwards, and the cycle repeats itself. It is peculiar that the mechanism for one of the commonest tachyarrhythmias is in part related to a 'boring old' block!
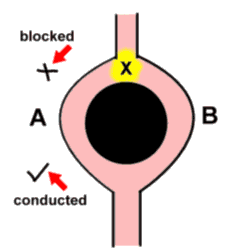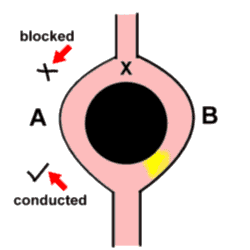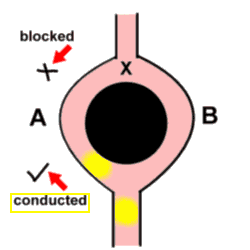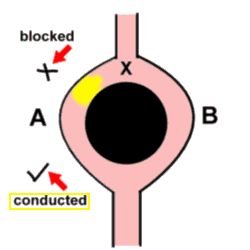
Note (and this is vital) that the initial block of the impulse as it tried to move down limb A might be related to say the local peculiarities of the conducting tissue, but frequently this block is simply related to timing - limb A is not quite ready to receive another impulse, thank you very much!
Also note that the heart is a dynamic, changing tissue. Although there is an inherent risk in this dynamism (something can go wrong) often the variablity in the heart is also its salvation (something that is wrong can also 'spontaneously' go right). As we will discover, doctors have probably accidentally killed an awful lot of people in discovering this simple principle! Two examples are perhaps in order - we know that there is normally a small, chaotic variability in the heart rhythm, and that just prior to developing an episode of life-threatening ventricular tachycardia, the heart often loses this variability and starts to beat with metronome-like regularity. Secondly, we know from the CAST study that 'Class IC antiarrhythmics' such as encainide, flecainide and propafenone, despite decreasing the frequency with which arrhythmias occur, appear to 'lock' those that do occur into a life-threatening perpetuation!
Also note that because blocks are often transient and dependent on timing, they need not even occupy a fixed anatomical location. Such a state appears to prevail in at least some types of atrial fibrillation, where we have multiple 'wavelets' moving around, propagating themselves and combining.
Increased automaticity is easily understood. We know that certain normal cardiac tissues have an inbuilt tendency to spontaneously depolarise - it doesn't take much imagination to realise that similar mechanisms might occur in other normally subservient muscle, and result in 'ectopic' activity. This might occur following a variety of insults - local ischaemia, hypokalaemia, or drugs such as digoxin, or indeed a combination of such factors.
Triggered activity is a bit more special. The key concept here is the 'afterdepolarisation' - after a normal action potential, the cellular transmembrane potential suddenly swings positive again, and if the timing and magnitude of this upswing is sufficient, a full depolarisation may occur again. And again. And again! There are (at least) two different mechanisms of triggered activity and these result in
- Early afterdepolarisations (EADs); or
- Delayed afterdepolarisations (DADs)
There are no rules that forbid a combination of the above to occur. For example, in bad old monomorphic ventricular tachycardia, the substrate for a re-entrant pathway might be a small myocardial scar (for example, following a previous myocardial infarction). An extrasystole occurring due to one of the other mechanisms might encounter the tissue surrounding this scar in just the right state of refractoriness to kick off a re-entrant circuit, which may then be perpetuated.
Membrane ion channels and their control
Excitable tissue works because it contains voltage-gated ion channels . We know that there is normally an electrical potential of about -70mV or more across cell-membranes, related to the difference in ionic concentrations across the membrane. If this voltage is made less negative, a point is eventually reached where the ion channels snap open (i.e. they are 'voltage gated'). This allows a massive influx of sodium or calcium ions, making the transmembrane potential even more positive - a classic example of positive feedback.
Fortunately, the same trigger that set off the opening of the channels soon results in their closing, and activation of a host of other mechanisms that finally restore the cell membrane to its pristine state. If we look at things from an evolutionary perspective, it seems that the first voltage-gated ion channel was a calcium channel, and that sodium channels (which are faster) and potassium channels (which are very special) diverged subsequently. This divergence is mirrored in the different expression of channels (as evolution seldom throws things away) - fast conducting tissues such as Purkinje fibres rely on sodium channels, while cells that need to conduct slowly (such as AV nodal cells) use calcium channels.
| Ion channels and membrane pumps | |
| Inward currents | |
| Current | Mechanism |
| I Na | An inward voltage-gated channel, the main driver of rapid depolarisation, the current only lasting about 1ms. (I Na-B may play a role in background current within SA nodal cells ?) A small sub-population of sodium channels continue to open during the action plateau! |
| I Ca-L | The main cardiac calcium channel, found in most heart cells, contributing to depolarisation of SA and AV nodal cells, and to the plateau of atrial, His-Purkinje and ventricular cells. Blocked by verapamil, diltiazem and dihydropyridines |
| I Ca-T | Occurs in pacemaker tissues, possibly aiding the later part of spontaneous depolarisation of atrial pacemaker tissue |
| I NS | This nonselective cation transporter is gated by the intracellular concentration of Ca ++ |
| I f | A non-specific cation transporter activated by negative membrane potentials, thus perhaps contributing to phase 4 depolarisation in AV nodal and His-Purkinje cells. |
| Outward currents | |
| I K1 | In the resting state this small current is active, turning off during depolarisation and then back on again during repolarisation. Absent from atrial pacemaker cells! |
| I Kr | The rapid component of the "delayed rectifier" that causes repolarisation of the membrane. Inhibited by drugs such as dofetilide and amiodarone. There is also an I Kur ultrarapid component of I K . |
| I Ks | The slow component of I K - inhibited by amiodarone and azimilide |
| I K(Ach) | A current activated following muscarinic (M 2 ) receptor stimulation. Also activated by adenosine. |
| I K(ATP) | Normally blocked by ATP, this channel opens up during ischaemia! |
| I K(Ca) | (Of questionable significance) |
| I to | A transient current that turns on after depolarisation, and then soon turns off again - may contribute to repolarisation heterogeneity! There may be two components (I to1 and I to2 ). |
| I Cl | (A small repolarising current that is enhanced by adrenergic stimulation) |
| Pumps | |
| I Na-K pump | An electrogenic pump that generates a constant, tiny outward current |
| I Na/Ca | The current generated by this pump is variable, depending on relative concentrations of Na + and Ca ++ inside and outside the cell. |
In order to restore things to their original state after a membrane depolarisation, the ions that moved across the membrane have to be moved back. A variety of active (energy-requiring) pumps exist to do this work.
The control of channel opening, closing and conductance is clearly vital, and intricate. Many drugs can be used to manipulate these channels either directly or indirectly. And one of our main traditional ways of looking at anti-arrhythmic drugs relies on the concept of blockade of these channels.
The Vaughan-Williams Classification
The much-maligned Vaughan-Williams classification of anti-arrhythmic drugs is still going strong. It has the virtue of simplicity:
| The Vaughan-Williams classification of anti-arrhythmic drugs | |
| Class | Mechanism of action |
| I | Sodium channel blockade |
| II | Beta adrenergic blockade |
| III | Prolongation of repolarisation ('Membrane stabilisation', often mainly due to potassium channel blockade) |
| IV | Calcium channel blockade |
See how three of the mechanisms are directly related to interference with ion channels. Not content with the above simplicity, the Class I agents are subdivided into three (IA, IB and IC) depending on the effect of the agents on their precise effects on depolarisation and repolarisation - IA slow depolarisation and conduction, and prolong repolarisation, IB have little effect on phase 0 in normal fibres and shorten repolarisation, and IC have little effect on repolarisation but profoundly depress both phase 0 depolarisation and conduction. This subclassification matters little, as they are all pretty nasty drugs!
A lot of fuss has been made about "state-dependent block" of ion channels by various drugs (especially sodium channel blockers) - the example always quoted is of lignocaine (which has a far greater affinity for activated sodium channels, and 'lets go' rapidly with a recovery time constant of under 1s once the channel is inactivated), and flecainide which blocks about the same number of channels in both systole and diastole owing to its recovery time constant of over ten seconds. The clinical relevance of all this palpitation is far from clear.
The Sicilian Gambit (declined)
In late 1990, the Task Force of the Working Group on Arrhythmias of the European Society of Cardiology (whew) got together in Taormina and decided to revolutionise arrhythmia assessment. They called this the "Sicilian Gambit". It hasn't yet quite caught on. The participants' cardiology abilities seem better than their chess knowledge (a 'gambit' in chess is a pawn sacrifice, and it's not immediately clear which pawn is being sacrificed - perhaps the patient - or what arrhythmia classification has to do with the Sicilian opening - ah - Taormina is in Sicily).
The paper describing the SG is however worth reading (Circulation 1991 October 84.4 1831-51). The authors make a valid criticism of the Vaughan-Williams classification on several grounds (It's a mix of different mechanisms, many drugs fall into multiple classes for example amiodarone, effects of agents might differ depending on the underlying disease process, and its incomplete - leaving out cholinergic agonists, adenosine, digoxin, alpha blockers, and so on). They propose a 'framework' incorporating knowledge of action of the agents, mechanisms of arrhythmia, and the "vulnerable parameter" (target) that we hope to modify using a drug.
They break the discussion into three - first considering the molecular and cellular targets of drug action, then how drugs affect the mechanisms of arrhythmias, and finally the clinical context of such drug use:
- Targets
The different cell membrane channels, pumps and receptors are well- discussed (brilliantly, for 1990). They go into considerable detail about the effects of- beta adrenergic receptor stimulation (modulation via G s of L-type calcium channels, I f , some K channels, I Cl and even on occasion, sodium channels; effects on L-type Ca channels might trigger EADs, or even DADs);
- alpha adrenergic receptor stimulation (with speculative mechanisms for arrhythmia promotion);
- M 2 muscarinic receptors (which are coupled to I K(ACh) and may also inhibit adenyl cyclase via G i ); and
- ) purinergic A 1 receptors (which modulate mainly AV nodal impulse conduction.)
- Mechanisms
The authors regard the 'vulnerable parameter' as that single alteration in electrophysiology that will terminate the arrhythmia (or prevent its initiation) with minimal adverse effect. For increased normal automaticity the vulnerable parameter is phase 4 depolarisation, and I f is important, although modulating this is complex. With abnormal automaticity I f becomes less important - as the maximum negative diastolic potential drops to under about 50 mV I Ca becomes predominant, and the vulnerable parameter should be the reduced maximum diastolic potential, perhaps correctable by increasing K + efflux or Na/K pump stimulation, or by attacking phase 4 depolarisation. Triggered activity is complex - EADs are related to prolonged action potentials, rationally managed by shortening the action potential duration; DADs, commonly found with intracellular calcium overload are logically treated using calcium channel blockers, or perhaps by blocking I NS or increasing potassium conductance. Reentry is commonly managed by blocking conduction or increasing refractoriness. - Clinical use The authors provide a complex table documenting the arrhythmia, mechanism, vulnerable parameter and representative drugs that might be used. This table illustrates one of the main problems with the Sicilian gambit - to apply it we need to understand the genesis of arrhythmias better than we currently do!
Individual agents
Because of the complex actions of the various agents, doctors who prescribe them should have a detailed knowledge of each agent prescribed, and the suitability of such agents for the various arrhythmias. Here we simply mention a few trends. This discussion would be incomplete without mention of the apparent vast benefit of an implantable cardioverter-defibrillator over conventional drug therapy (e.g. MADIT and AVID).
- Adenosine has become extremely popular in the management of re-entrant supraventricular tachyarrhythmias. It activates I K(Ach), transiently (under 5s) increasing AV nodal refractoriness, and often terminating AV nodal reentrant tachyarrhythmias, where it's the drug of choice. Side effects are usually relatively minor (flushing, occasional precipitation of atrial fibrillation - the latter being a potential problem in the presence of a fast-conducting accessory pathway). The major problem is that if the underlying substrate has not been addressed, the arrhythmia often recurs.
- Class I agents have generally fallen into disfavour, notably in patients with underlying ischaemic heart disease, notably following the CAST study where mortality increased by 6%. Encainide and Propafenone are occasionally used for maintenance of sinus rhythm in patients with supra-ventricular arrhythmias such as atrial fibrillation provided there is no underlying structural heart disease. Similar, infrequently used agents with Class I actions are mexiletine, tocainide (which also causes bone marrow aplasia / lung fibrosis), and moricizine. Lignocaine is still useful for some acute ventricular arrhythmias, but its routine use in ischaemic heart disease must be strongly condemned.
- Amiodarone is becoming more popular, for both acute and chronic management of both ventricular and supra-ventricular arrhythmias. It is discussed in detail elsewhere. Disadvantages include cost (especially of the intravenous form) and the plethora of serious side effects seen with chronic use. Amiodarone has actions in all four Vaughan-Williams classes, but its predominant effect is Class III, due to block of both I Kr and I Ks . Despite the prolongation of action potential duration, torsades is distinctly unusual with amiodarone therapy. In the CHF-STAT trial there was no lowering of mortality with amiodarone, but in GESICA with a smaller sample size a 30% decrease in mortality was noted.
- A number of new class III agents have been approved or are in
the pipeline. These include dofetilide (which selectively blocks
I Kr , and has been used with about 30% success for conversion of
chronic atrial fibrillation, and sinus rhythm maintenace - see
the DIAMOND trial), and ibutilide (only available in an intravenous form,
used for conversion of atrial fibrillation). Ibutilide has effects on
both sodium channels and I Kr .
Most have the side effect of promoting the serious arrhythmia torsade de pointes, just like the other commonly used class III agent, sotalol. Sotalol is racemic, and the membrane-stabilising effects are seen at higher doses than the beta blockade. It's pretty safe with myocardial dysfunction, perhaps related to increased intracellular calcium levels improving contractility somewhat. The ESVEM study showed the superiority of sotalol over six different Class I agents. Unfortunately the enantiomer d-Sotalol has not lived up to expectation (nor did the patients treated with it - put to the SWORD, as it were)! - Digoxin (digitalis glycosides) has a long and chequered history, but the great wheel has again swung against it. A lot of the chronic effect of digoxin may be related to increased vagal tone! Disadvantages however include the low therapeutic index, high incidence of proarrhythmia, and the fact that it maintains atrial fibrillation rather than converting it to sinus rhythm (which is generally now considered far more desirable). Even where digoxin is used for rate control in AF, the rate often goes crazy once the person exercises!
- Bretylium is an unusual drug with a variety of complex actions, initially releasing noradrenaline and then apparently blocking potassium channels. It has always seemed to be an attractive drug because it may lower defibrillation threshold in the fibrillating heart, but its role is far from clear - too often physicians seem to administer it as the 'last rites'!
- Other agents:
- Calcium channel blockers have fallen into disfavour for management of supraventricular arrhythmias due to their negative inotropic effects, and the odd misguided doctor who kills patients with ventricular tachycardia by administering verapamil. For SVT, try adenosine.
- Quinidine has both Class I and potassium-channel blocking effects, and is occasionally used for maintenance of sinus rhythm in atrial flutter or fibrillation. It also causes alpha blockade and vagolysis.
- intravenous Magnesium is regarded by many as the drug of choice for torsade de pointes.
- Procainamide is similar to quinine (without the alpha blockade and vagolysis), is rapidly eliminated (necessitating use of slow-release formulations), and causes lupus syndrome in slow acetylators. Enough said.
- Disopyramide is like quinidine but is anticholinergic, aninotropic and may precipitate torsade de pointes.
Molecular Genetics - the long QT syndrome
Of great interest is the recent delineation of at least four different hereditary syndromes characterised by myocardial ion channel abnormalities. Clinically these manifest as a prolonged QT interval on surface ECG, associated with early sudden death in some affected individuals. Identified gene abnormalities include:
- KVLQT1 (Chromosome 11) - probably a component of I Ks ;
- HERG (Chromosome 7) - which codes for the I Kr channel;
- SCN5A (Chromosome 3) - coding for a sodium channel; and
- MinK - together with KVLQT1, a component of I Ks .
References
- ESC Task Force The Sicilian Gambit Circulation 1991 84 1831-51.
- Roden DM Antiarrhythmic Drugs in Hardman JG et al. Goodman & Gilman's The pharmacological basis of therapeutics 9ed. McGraw Hill 1996. ISBN 007 0262667.
- CAST investigators Preliminary report : effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction NEJM 1989 321 406-12.
- Waldo et al. {SWORD} Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction Lancet 1996 348 7-12.
- Mason {ESVEM}. A comparison of seven antiarrhythmic drugs in patients with ventricular tachyarrhythmias . NEJM 1993 329 452-8.
- Singh SN et al. {CHF-STAT} Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmias NEJM 1995 333 77-2.
- Doval HC et al. {GESICA}. Randomized trial of low-dose amiodarone in severe congestive heart failure Lancet 1994 344 493-8.
- Moss AJ et al. {MADIT}. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia NEJM 1996 335 1933-0.
- Grant AO. Mechanisms of atrial fibrillation and action of drugs used in its management AJC 1998 82 43N-9N.
- Olsson SB. Atrial fibrillation - new aspects on mechanism and treatment J Int Med 1996 239 3-15.
- Keating MT & Sanguinetti MC. Molecular genetic insights into cardiovascular disease Science 1996 272 681-5.